This is a preprint.
Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant
- PMID: 36263060
- PMCID: PMC9580377
- DOI: 10.1101/2022.09.12.507614
Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant
Update in
-
Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice.Nat Med. 2023 Jan;29(1):247-257. doi: 10.1038/s41591-022-02092-8. Epub 2022 Oct 20. Nat Med. 2023. PMID: 36265510 Free PMC article.
Abstract
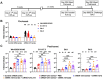
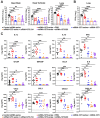
The emergence of SARS-CoV-2 variants in the Omicron lineage with large numbers of substitutions in the spike protein that can evade antibody neutralization has resulted in diminished vaccine efficacy and persistent transmission. One strategy to broaden vaccine-induced immunity is to administer bivalent vaccines that encode for spike proteins from both historical and newly-emerged variant strains. Here, we evaluated the immunogenicity and protective efficacy of two bivalent vaccines that recently were authorized for use in Europe and the United States and contain two mRNAs encoding Wuhan-1 and either BA.1 (mRNA-1273.214) or BA.4/5 (mRNA-1273.222) spike proteins. As a primary immunization series in BALB/c mice, both bivalent vaccines induced broader neutralizing antibody responses than the constituent monovalent vaccines (mRNA-1273 [Wuhan-1], mRNA-1273.529 [BA.1], and mRNA-1273-045 [BA.4/5]). When administered to K18-hACE2 transgenic mice as a booster at 7 months after the primary vaccination series with mRNA-1273, the bivalent vaccines induced greater breadth and magnitude of neutralizing antibodies compared to an mRNA-1273 booster. Moreover, the response in bivalent vaccine-boosted mice was associated with increased protection against BA.5 infection and inflammation in the lung. Thus, boosting with bivalent Omicron-based mRNA-1273.214 or mRNA-1273.222 vaccines enhances immunogenicity and protection against currently circulating SARS-CoV-2 strains.
Figures


References
-
- Qi H., Liu B., Wang X. & Zhang L. The humoral response and antibodies against SARS-CoV-2 infection. Nat Immunol 23, 1008–1020 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
