Heart Transplant Immunosuppression Strategies at Cedars-Sinai Medical Center
- PMID: 36263111
- PMCID: PMC9536714
- DOI: 10.36628/ijhf.2020.0034
Heart Transplant Immunosuppression Strategies at Cedars-Sinai Medical Center
Abstract
Heart transplant is the optimal treatment for selected patients with end-stage heart failure. Immunosuppression after heart transplantation has significantly reduced the incidence of rejection and improved patient outcomes with the routine use of calcineurin inhibitors. Antimetabolites and proliferation signal inhibitors add to the improvement in patient outcomes as well. The goal of induction therapy is to provide intense immunosuppression when the risk of allograft rejection is highest. Most maintenance immunosuppressive protocols employ a 3-drug regimen consisting of a calcineurin inhibitor, an antimetabolite agent and glucocorticoids. The management of rejection proceeds in a stepwise fashion based on the severity of rejection detected on biopsy and the patient's clinical presentation. This review will cover induction, maintenance, rejection therapy and some special considerations including sensitization, renal sparing protocol, and corticosteroid weaning. It will end in consideration of potential future directions in immunosuppressive strategies to promote patient and graft survival.
Keywords: Desensitization, immunologic; Graft rejection; Heart transplantation; Immunosuppression; Maintenance.
Copyright © 2021. Korean Society of Heart Failure.
Conflict of interest statement
Conflict of Interest: D.H.C. received research grants from Amgen, Biocardia, and Mesoblast and has moderate stock interest in Abbot Laboratories, Abbvie Inc, Repligen Corporation, Amarin Corporation and Portola Pharmaceuticals. J.K.P. received research grants from Alexion Pharmaceuticals, Pfizer, Alnylam Pharmaceuticals and Astra Zeneca. J.A.K. received research grants from CareDx Inc., Sanofi-Genzyme and CSL-Behringer. All other authors have no conflicts of interest to disclose.
Figures
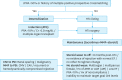

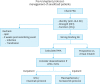
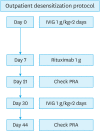

References
-
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1056–1066. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

