Real-world effectiveness and persistence of reference etanercept versus biosimilar etanercept GP2015 among rheumatoid arthritis patients: A cohort study
- PMID: 36263118
- PMCID: PMC9575986
- DOI: 10.3389/fphar.2022.980832
Real-world effectiveness and persistence of reference etanercept versus biosimilar etanercept GP2015 among rheumatoid arthritis patients: A cohort study
Abstract
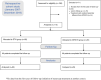
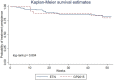
Although several randomized clinical trials have confirmed that there is no difference in efficacy between etanercept and its biosimilar versions in the treatment of rheumatoid arthritis (RA), limited real-world evidence is available. We conducted a cohort study to compare the effectiveness and treatment persistence between the reference etanercept (ETN) and the biosimilar GP2015 in RA patients in a real-life setting. Adults with a diagnosis of RA who initiated treatment with ETN or GP2015, between January 2007 and December 2019, were included. The follow-up period was 52 weeks. The primary outcome was the mean of change in the DAS28-CRP values and the adjusted mean difference from baseline to 52 weeks between ETN and GP2015. Other effectiveness endpoints assessed were the rate of patients who achieved remission or low disease activity (LDA) at week 52, who showed a reduction of DAS28-CRP value greater than or equal to 1.2 from baseline to week 52 and rate of good responder patients (those meeting both effectiveness measures) at week 52. Treatment effectiveness over time (baseline, 26 and 52 weeks) was compared between the ETN and GP2015 groups using mixed effects models. Treatment persistence (probability of maintaining the same treatment over time) was also evaluated and shown using Kaplan-Meier survival curves. A total of 115 RA patients were included (ETN, n = 90; GP2015, n = 25). No differences were observed in the primary outcome: DAS28-CRP score decreased from baseline to week 52 [5.1 to 2.7 (mean of change -2.37) in ETN group and 5.0 to 2.2 (mean of change -2.84) in GP2015 group, p-value = 0.372] and the adjusted mean difference was -0.37 (-1.03 to 0.29). No differences were also observed in the other effectiveness endpoints assessed among patients treated with ETN or GP2015: rate of patients who achieved remission (54.1% vs. 66.7%, p-value = 0.303) and LDA (71.6% vs. 80.9%, p-value = 0.391) at week 52, reduction of DAS28-CRP value greater than or equal to 1.2 from baseline to week 52 (75.6% vs. 80.9%, p-value = 0.613) and rate of good responder patients (58.1% vs. 76.1%, p-value = 0.202). Drug survival was 82% and 80% for ETN and GP2015, respectively (log-rank p-value = 0.804). Etanercept and its biosimilar GP2015 show similar effectiveness and treatment persistence in RA patients in a real-life setting.
Keywords: GP2015; biosimilar agents; drug survival real-life data; effectivenes; etanercept (enbrel); rheumatoid anhritis.
Copyright © 2022 Carballo, Pérez García, Grau, Monfort, Durán-Jordà, Echeverría-Esnal and Ferrández.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Efficacy, safety and immunogenicity of GP2015, an etanercept biosimilar, compared with the reference etanercept in patients with moderate-to-severe rheumatoid arthritis: 24-week results from the comparative phase III, randomised, double-blind EQUIRA study.RMD Open. 2018 Nov 14;4(2):e000757. doi: 10.1136/rmdopen-2018-000757. eCollection 2018. RMD Open. 2018. PMID: 30487998 Free PMC article.
-
Switch from reference etanercept to SDZ ETN, an etanercept biosimilar, does not impact efficacy, safety, and immunogenicity of etanercept in patients with moderate-to-severe rheumatoid arthritis: 48-week results from the phase III, randomized, double-blind EQUIRA study.Arthritis Res Ther. 2019 May 28;21(1):130. doi: 10.1186/s13075-019-1907-x. Arthritis Res Ther. 2019. PMID: 31138316 Free PMC article. Clinical Trial.
-
The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis.Br J Dermatol. 2017 Apr;176(4):928-938. doi: 10.1111/bjd.15152. Epub 2017 Mar 1. Br J Dermatol. 2017. PMID: 27787890 Clinical Trial.
-
Characterization and non-clinical assessment of the proposed etanercept biosimilar GP2015 with originator etanercept (Enbrel(®)).Expert Opin Biol Ther. 2016 Oct;16(10):1185-95. doi: 10.1080/14712598.2016.1217329. Epub 2016 Aug 16. Expert Opin Biol Ther. 2016. PMID: 27463856 Review.
-
GP2015: An Etanercept Biosimilar.BioDrugs. 2017 Dec;31(6):555-558. doi: 10.1007/s40259-017-0246-1. BioDrugs. 2017. PMID: 28940172 Free PMC article. Review.
Cited by
-
Disease remission and sustained remission after etanercept biosimilar or originator initiation in rheumatoid arthritis: an interim real-world analysis.Arthritis Res Ther. 2025 Jul 17;27(1):150. doi: 10.1186/s13075-025-03607-7. Arthritis Res Ther. 2025. PMID: 40676646 Free PMC article.
-
Comparative effectiveness and safety of biosimilars versus reference biologics in rheumatoid arthritis during treatment initiation: a systematic review of real-world evidence.Int J Clin Pharm. 2025 Jun 25. doi: 10.1007/s11096-025-01956-6. Online ahead of print. Int J Clin Pharm. 2025. PMID: 40560336 Review.
-
Persistence and safety of anti-TNF biosimilars versus originators in immune-mediated inflammatory diseases: an observational study on the French National Health Data System.RMD Open. 2024 Mar 6;10(1):e003531. doi: 10.1136/rmdopen-2023-003531. RMD Open. 2024. PMID: 38453213 Free PMC article.
-
Treatment Persistence in Rheumatoid Arthritis Before and After Etanercept Biosimilar Introduction: A Nationwide Australian Real-World Study.Pharmaceut Med. 2025 Jul 16. doi: 10.1007/s40290-025-00577-8. Online ahead of print. Pharmaceut Med. 2025. PMID: 40670897
References
-
- Aaltonen K. J., Ylikylä S., Joensuu J. T., Isomäki P., Pirilä L., Kauppi M., et al. (2017). Efficacy and effectiveness of tumour necrosis factor inhibitors in the treatment of rheumatoid arthritis in randomized controlled trials and routine clinical practice. Rheumatology 56 (5), 725–735. 10.1093/rheumatology/kew467 - DOI - PubMed
-
- Aletaha D., Neogi T., Silman A. J., Funovits J., Felson D. T., Bingham C. O., et al. (2010). 2010 rheumatoid arthritis classification criteria: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 69 (9), 1580–1588. 10.1136/ard.2010.138461 - DOI - PubMed
-
- Atzeni F., Gerratana E., Bongiovanni S., Talotta R., Miceli G., Salaffi F., et al. (2021). Efficacy and safety of biosimilar and originator etanercept in rheumatoid arthritis patients: Real-life data. Isr. Med. Assoc. J. 23 (7), 344–349. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

