The role of immune checkpoints in cardiovascular disease
- PMID: 36263134
- PMCID: PMC9574006
- DOI: 10.3389/fphar.2022.989431
The role of immune checkpoints in cardiovascular disease
Abstract
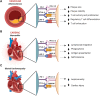
Immune checkpoint inhibitors (ICI) are monoclonal antibodies which bind to immune checkpoints (IC) and their ligands to prevent inhibition of T-cell activation by tumor cells. Currently, multiple ICI are approved targeting Cytotoxic T-lymphocyte antigen 4 (CTLA-4), Programmed Death Protein 1 (PD-1) and its ligand PD-L1, and Lymphocyte-activation gene 3 (LAG-3). This therapy has provided potent anti-tumor effects and improved prognosis for many cancer patients. However, due to systemic effects, patients can develop immune related adverse events (irAE), including possible life threatening cardiovascular irAE, like atherosclerosis, myocarditis and cardiomyopathy. Inhibition of vascular IC is associated with increased atherosclerotic burden and plaque instability. IC protect against atherosclerosis by inhibiting T-cell activity and cytokine production, promoting regulatory T-cell differentiation and inducing T-cell exhaustion. In addition, PD-L1 on endothelial cells might promote plaque stability by reducing apoptosis and increasing expression of tight junction molecules. In the heart, IC downregulate the immune response to protect against cardiac injury by reducing T-cell activity and migration. Here, inhibition of IC could induce life-threatening T-cell-mediated-myocarditis. One proposed purpose behind lymphocyte infiltration is reaction to cardiac antigens, caused by decreased self-tolerance, and thereby increased autoimmunity because of IC inhibition. In addition, there are several reports of ICI-mediated cardiomyopathy with immunoglobulin G expression on cardiomyocytes, indicating an autoimmune response. IC are mostly known due to their cardiotoxicity. However, t his review compiles current knowledge on mechanisms behind IC function in cardiovascular disease with the aim of providing an overview of possible therapeutic targets in prevention or treatment of cardiovascular irAEs.
Keywords: CTLA-4; LAG-3; PD-1; atherosclerosis; cardiomyopathy; immune checkpoint inhibitors; immune checkpoints; myocarditis.
Copyright © 2022 Yousif, Tanja, de Boer, Teske and Meijers.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Andres M., Ramalingam S. (2019). Cardiovascular complications of immune checkpoint inhibitors, TILs and CAR T-cell therapies. escardio.org. Available at: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volum... (Accessed July 5, 2022).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

