Efficacy and safety of total glucosides of paeony as an add-on treatment in adolescents and adults with chronic urticaria: A systematic review and meta-analysis
- PMID: 36263138
- PMCID: PMC9574670
- DOI: 10.3389/fphar.2022.961371
Efficacy and safety of total glucosides of paeony as an add-on treatment in adolescents and adults with chronic urticaria: A systematic review and meta-analysis
Abstract
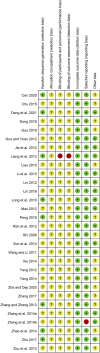
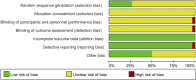
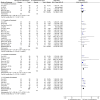
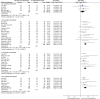
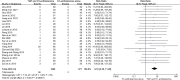
Background: Total glycosides of paeony (TGP), an active compound extracted from the dried roots of Paenoia lactiflora Pall., has been widely used to treat chronic urticaria (CU) in China. This study aims to systematically evaluate the efficacy and safety of TGP as an add-on treatment for the treatment of CU in adolescents and adults. Methods: Eight literature databases and two clinical trial registries were searched from their inception to 31 May 2022. Randomized controlled trials on TGP as an add-on treatment for CU in adolescents and adults were included. The Cochrane Collaboration's risk of bias tool was used for the methodological quality assessment, and RevMan 5.3 software and Stata 12.0 software were used for data analyses. Results: A total of 30 studies with 2,973 participants were included in this meta-analysis. The methodological qualities of all included studies were suboptimal. The pooled results showed that TGP combined with H1-antihistamine was superior to H1-antihistamine alone in the cure rate (risk ratio (RR) = 1.54, 95% confidence interval (CI) = 1.39 to 1.71, p < 0.00001), total efficacy rate (RR = 1.33, 95%CI = 1.26 to 1.40, p < 0.00001), urticaria activity score 7 (mean difference (MD) = -4.03, 95%CI = -6.62 to -1.44, p = 0.002), recurrence rate (RR = 0.31, 95%CI = 0.20 to 0.46, p < 0.00001), and the level of IgE in serum (standardized mean difference (SMD) = -1.96, 95%CI = -3.02 to -0.90, p = 0.0003). In terms of safety, the incidence of diarrhea (RR = 6.19, 95%CI = 3.39 to 11.29, p < 0.00001) was significantly increased in the TGP plus H1-antihistamine groups, and no abnormal results of laboratory tests and electrocardiogram were reported in two groups. The qualities of evidences were evaluated as moderate to low. Conclusions: TGP as an add-on treatment could provide a good effect for CU in adolescents and adults with mild and tolerable adverse events. However, in view of poor methodological quality, high-quality and long-term clinical trials are needed in the future to confirm and update the evidence.
Keywords: chronic urticaria; efficacy; meta-analysis; randomized controlled trial; safety; total glucosides of paeony.
Copyright © 2022 Li, Li, Xiang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



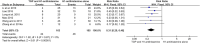
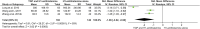

Similar articles
-
Total Glucosides of Paeonia lactiflora for Safely Reducing Disease Activity in Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis.Front Pharmacol. 2022 Jan 31;13:834947. doi: 10.3389/fphar.2022.834947. eCollection 2022. Front Pharmacol. 2022. PMID: 35173622 Free PMC article. Review.
-
Clinical safety of total glucosides of paeony adjuvant therapy for rheumatoid arthritis treatment: a systematic review and meta-analysis.BMC Complement Med Ther. 2021 Mar 26;21(1):102. doi: 10.1186/s12906-021-03252-y. BMC Complement Med Ther. 2021. PMID: 33771151 Free PMC article.
-
Add-on effects of total glucosides of paeony on conventional therapies for psoriasis: a systematic review and meta-analysis of randomized controlled trials.Front Pharmacol. 2025 Feb 19;16:1527288. doi: 10.3389/fphar.2025.1527288. eCollection 2025. Front Pharmacol. 2025. PMID: 40046738 Free PMC article.
-
Efficacy and safety of Tripterygium glycosides as an add-on treatment in adults with chronic urticaria: a systematic review and meta-analysis.Pharm Biol. 2023 Dec;61(1):324-336. doi: 10.1080/13880209.2023.2169468. Pharm Biol. 2023. PMID: 36694954 Free PMC article.
-
Efficacy and safety of total glucosides of paeony in the treatment of systemic lupus erythematosus: A systematic review and meta-analysis.Front Pharmacol. 2022 Dec 7;13:932874. doi: 10.3389/fphar.2022.932874. eCollection 2022. Front Pharmacol. 2022. PMID: 36569311 Free PMC article.
Cited by
-
Therapeutic potential and pharmacological insights of total glucosides of paeony in dermatologic diseases: a comprehensive review.Front Pharmacol. 2025 Jan 2;15:1423717. doi: 10.3389/fphar.2024.1423717. eCollection 2024. Front Pharmacol. 2025. PMID: 39822741 Free PMC article. Review.
-
A Review of the Potential Benefits of Herbal Medicines, Small Molecules of Natural Sources, and Supplements for Health Promotion in Lupus Conditions.Life (Basel). 2023 Jul 19;13(7):1589. doi: 10.3390/life13071589. Life (Basel). 2023. PMID: 37511964 Free PMC article. Review.
References
-
- Cen W. D. (2020). Efficacy and safety of desloratadine citrate disodium combined with total glucosides of paeony capsule for chronic urticaria. Electron. J. Clin. Med. Lit. 7 (67), 151+156.
-
- Chu M. Q. (2015). Efficacy of total glucosides of peony combined with acrivastine for chronic urticaria. Chin. J. Lepr. Skin. Dis. 31 (7), 431–432.
-
- Deng Y., Jiang Y. H., Wan Y., Yang H. R., Yu C. S. (2021). Lupatadine combined with total glucosides of peony in the treatment of chronic idiopathic urticaria. World. Lat. Med. Inf. Electron. Vers. 21 (84), 171–173. 10.3969/j.issn.1671-3141.2021.84.050 - DOI
-
- Dong S. Y. (2016). Analysis of effect of total glucosides of peony combined with desloratadine citrate disodium for the treatment of chronic urticaria. J. Aerosp. Med. 27 (3), 295–296. 10.3969/j.issn.2095-1434.2016.03.012 - DOI
Publication types
LinkOut - more resources
Full Text Sources

