Pyroptotic cell death in SARS-CoV-2 infection: revealing its roles during the immunopathogenesis of COVID-19
- PMID: 36263178
- PMCID: PMC9576507
- DOI: 10.7150/ijbs.77561
Pyroptotic cell death in SARS-CoV-2 infection: revealing its roles during the immunopathogenesis of COVID-19
Abstract
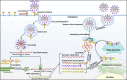
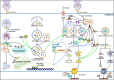
The rapid dissemination of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), remains a global public health emergency. The host immune response to SARS-CoV-2 plays a key role in COVID-19 pathogenesis. SARS-CoV-2 can induce aberrant and excessive immune responses, leading to cytokine storm syndrome, autoimmunity, lymphopenia, neutrophilia and dysfunction of monocytes and macrophages. Pyroptosis, a proinflammatory form of programmed cell death, acts as a host defense mechanism against infections. Pyroptosis deprives the replicative niche of SARS-CoV-2 by inducing the lysis of infected cells and exposing the virus to extracellular immune attack. Notably, SARS-CoV-2 has evolved sophisticated mechanisms to hijack this cell death mode for its own survival, propagation and shedding. SARS-CoV-2-encoded viral products act to modulate various key components in the pyroptosis pathways, including inflammasomes, caspases and gasdermins. SARS-CoV-2-induced pyroptosis contriubtes to the development of COVID-19-associated immunopathologies through leakage of intracellular contents, disruption of immune system homeostasis or exacerbation of inflammation. Therefore, pyroptosis has emerged as an important mechanism involved in COVID-19 immunopathogenesis. However, the entangled links between pyroptosis and SARS-CoV-2 pathogenesis lack systematic clarification. In this review, we briefly summarize the characteristics of SARS-CoV-2 and COVID-19-related immunopathologies. Moreover, we present an overview of the interplay between SARS-CoV-2 infection and pyroptosis and highlight recent research advances in the understanding of the mechanisms responsible for the implication of the pyroptosis pathways in COVID-19 pathogenesis, which will provide informative inspirations and new directions for further investigation and clinical practice. Finally, we discuss the potential value of pyroptosis as a therapeutic target in COVID-19. An in-depth discussion of the underlying mechanisms of COVID-19 pathogenesis will be conducive to the identification of potential therapeutic targets and the exploration of effective treatment measures aimed at conquering SARS-CoV-2-induced COVID-19.
Keywords: COVID-19; SARS-CoV-2; cytokine storm; immunopathogenesis; inflammasomes; pyroptosis; therapeutic targets.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

