Comparative analysis of extracellular vesicle isolation methods from human AML bone marrow cells and AML cell lines
- PMID: 36263223
- PMCID: PMC9574064
- DOI: 10.3389/fonc.2022.949261
Comparative analysis of extracellular vesicle isolation methods from human AML bone marrow cells and AML cell lines
Abstract
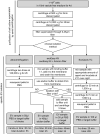
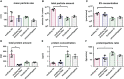
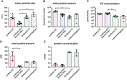
Cellular crosstalk between hematopoietic stem/progenitor cells and the bone marrow (BM) niche is vital for the development and maintenance of myeloid malignancies. These compartments can communicate via bidirectional transfer of extracellular vesicles (EVs). EV trafficking in acute myeloid leukemia (AML) plays a crucial role in shaping the BM microenvironment into a leukemia-permissive niche. Although several EV isolation methods have been developed, it remains a major challenge to define the most accurate and reliable procedure. Here, we tested the efficacy and functional assay compatibility of four different EV isolation methods in leukemia-derived EVs: (1) membrane affinity-based: exoEasy Kit alone and (2) in combination with Amicon filtration; (3) precipitation: ExoQuick-TC; and (4) ultracentrifugation (UC). Western blot analysis of EV fractions showed the highest enrichment of EV marker expression (e.g., CD63, HSP70, and TSG101) by precipitation with removal of overabundant soluble proteins [e.g., bovine serum albumin (BSA)], which were not discarded using UC. Besides the presence of damaged EVs after UC, intact EVs were successfully isolated with all methods as evidenced by highly maintained spherical- and cup-shaped vesicles in transmission electron microscopy. Nanoparticle tracking analysis of EV particle size and concentration revealed significant differences in EV isolation efficacy, with exoEasy Kit providing the highest EV yield recovery. Of note, functional assays with exoEasy Kit-isolated EVs showed significant toxicity towards treated target cells [e.g., mesenchymal stromal cells (MSCs)], which was abrogated when combining exoEasy Kit with Amicon filtration. Additionally, MSC treated with green fluorescent protein (GFP)-tagged exoEasy Kit-isolated EVs did not show any EV uptake, while EV isolation by precipitation demonstrated efficient EV internalization. Taken together, the choice of EV isolation procedure significantly impacts the yield and potential functionality of leukemia-derived EVs. The cheapest method (UC) resulted in contaminated and destructed EV fractions, while the isolation method with the highest EV yield (exoEasy Kit) appeared to be incompatible with functional assays. We identified two methods (precipitation-based ExoQuick-TC and membrane affinity-based exoEasy Kit combined with Amicon filtration) yielding pure and intact EVs, also suitable for application in functional assays. This study highlights the importance of selecting the right EV isolation method depending on the desired experimental design.
Keywords: AML; EV isolation methods; bone marrow niche; extracellular vesicles; intercellular communication.
Copyright © 2022 Lang, Buck, Rivière, Stambouli, Sachenbacher, Choudhary, Dietz, Giebel, Bassermann, Oostendorp, Götze and Hecker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma.J Transl Med. 2018 Jan 9;16(1):1. doi: 10.1186/s12967-017-1374-6. J Transl Med. 2018. PMID: 29316942 Free PMC article.
-
Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin.J Extracell Vesicles. 2021 Jul;10(9):e12128. doi: 10.1002/jev2.12128. Epub 2021 Jul 22. J Extracell Vesicles. 2021. PMID: 34322205 Free PMC article.
-
Comparative analysis of extracellular vesicles isolated from human mesenchymal stem cells by different isolation methods and visualisation of their uptake.Exp Cell Res. 2022 May 15;414(2):113097. doi: 10.1016/j.yexcr.2022.113097. Epub 2022 Mar 9. Exp Cell Res. 2022. PMID: 35276207
-
Extracellular Vesicles and Chemotherapy Resistance in the AML Microenvironment.Front Oncol. 2020 Feb 14;10:90. doi: 10.3389/fonc.2020.00090. eCollection 2020. Front Oncol. 2020. PMID: 32117744 Free PMC article. Review.
-
Transforming the Niche: The Emerging Role of Extracellular Vesicles in Acute Myeloid Leukaemia Progression.Int J Mol Sci. 2024 Apr 17;25(8):4430. doi: 10.3390/ijms25084430. Int J Mol Sci. 2024. PMID: 38674015 Free PMC article. Review.
Cited by
-
Use of extracellular vesicle microRNA profiles in patients with acute myeloid leukemia for the identification of novel biomarkers.PLoS One. 2024 Aug 23;19(8):e0306962. doi: 10.1371/journal.pone.0306962. eCollection 2024. PLoS One. 2024. PMID: 39178208 Free PMC article.
-
Bifurcated Asymmetric Field Flow Fractionation of Nanoparticles in PDMS-Free Microfluidic Devices for Applications in Label-Free Extracellular Vesicle Separation.Polymers (Basel). 2023 Feb 4;15(4):789. doi: 10.3390/polym15040789. Polymers (Basel). 2023. PMID: 36850073 Free PMC article.
-
Emerging Roles of Using Small Extracellular Vesicles as an Anti-Cancer Drug.Int J Mol Sci. 2023 Sep 14;24(18):14063. doi: 10.3390/ijms241814063. Int J Mol Sci. 2023. PMID: 37762393 Free PMC article. Review.
-
Small extracellular vesicles (sEVs)-based gene delivery platform for cell-specific CRISPR/Cas9 genome editing.Theranostics. 2024 Apr 28;14(7):2777-2793. doi: 10.7150/thno.92133. eCollection 2024. Theranostics. 2024. PMID: 38773978 Free PMC article.
-
Enhancing Extracellular Vesicle Analysis by Integration of Large-Volume Sample Stacking in Capillary Electrophoresis with Asymmetrical Flow Field-Flow Fractionation.Anal Chem. 2023 Oct 24;95(42):15778-15785. doi: 10.1021/acs.analchem.3c03303. Epub 2023 Oct 5. Anal Chem. 2023. PMID: 37795969 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

