Exploring anti-androgen therapies in hormone dependent prostate cancer and new therapeutic routes for castration resistant prostate cancer
- PMID: 36263323
- PMCID: PMC9575553
- DOI: 10.3389/fendo.2022.1006101
Exploring anti-androgen therapies in hormone dependent prostate cancer and new therapeutic routes for castration resistant prostate cancer
Abstract
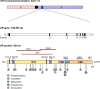
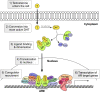
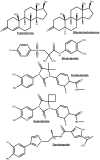
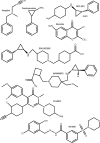
Androgen deprivation therapies (ADTs) are important treatments which inhibit androgen-induced prostate cancer (PCa) progression by either preventing androgen biosynthesis (e.g. abiraterone) or by antagonizing androgen receptor (AR) function (e.g. bicalutamide, enzalutamide, darolutamide). A major limitation of current ADTs is they often remain effective for limited durations after which patients commonly progress to a lethal and incurable form of PCa, called castration-resistant prostate cancer (CRPC) where the AR continues to orchestrate pro-oncogenic signalling. Indeed, the increasing numbers of ADT-related treatment-emergent neuroendocrine-like prostate cancers (NePC), which lack AR and are thus insensitive to ADT, represents a major therapeutic challenge. There is therefore an urgent need to better understand the mechanisms of AR action in hormone dependent disease and the progression to CRPC, to enable the development of new approaches to prevent, reverse or delay ADT-resistance. Interestingly the AR regulates distinct transcriptional networks in hormone dependent and CRPC, and this appears to be related to the aberrant function of key AR-epigenetic coregulator enzymes including the lysine demethylase 1 (LSD1/KDM1A). In this review we summarize the current best status of anti-androgen clinical trials, the potential for novel combination therapies and we explore recent advances in the development of novel epigenetic targeted therapies that may be relevant to prevent or reverse disease progression in patients with advanced CRPC.
Keywords: PARP inhibitors; Therapy; anti-androgen; castration resistant prostate cancer; epigenetic targeted treatment.
Copyright © 2022 Harris, Metzler, Lothion-Roy, Varun, Woodcock, Haigh, Endeley, Haque, Toss, Alsaleem, Persson, Gudas, Rakha, Robinson, Khani, Martin, Moyer, Brownlie, Madhusudan, Allegrucci, James, Rutland, Fray, Ntekim, de Brot, Mongan and Jeyapalan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

