Analyzing the mechanisms that facilitate the subtype-specific assembly of γ-aminobutyric acid type A receptors
- PMID: 36263376
- PMCID: PMC9574402
- DOI: 10.3389/fnmol.2022.1017404
Analyzing the mechanisms that facilitate the subtype-specific assembly of γ-aminobutyric acid type A receptors
Abstract
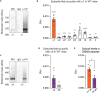
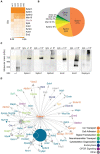
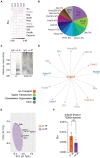
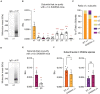
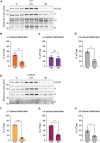
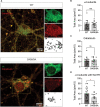
Impaired inhibitory signaling underlies the pathophysiology of many neuropsychiatric and neurodevelopmental disorders including autism spectrum disorders and epilepsy. Neuronal inhibition is regulated by synaptic and extrasynaptic γ-aminobutyric acid type A receptors (GABA A Rs), which mediate phasic and tonic inhibition, respectively. These two GABA A R subtypes differ in their function, ligand sensitivity, and physiological properties. Importantly, they contain different α subunit isoforms: synaptic GABA A Rs contain the α1-3 subunits whereas extrasynaptic GABA A Rs contain the α4-6 subunits. While the subunit composition is critical for the distinct roles of synaptic and extrasynaptic GABA A R subtypes in inhibition, the molecular mechanism of the subtype-specific assembly has not been elucidated. To address this issue, we purified endogenous α1- and α4-containing GABA A Rs from adult murine forebrains and examined their subunit composition and interacting proteins using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) and quantitative analysis. We found that the α1 and α4 subunits form separate populations of GABA A Rs and interact with distinct sets of binding proteins. We also discovered that the β3 subunit, which co-purifies with both the α1 and α4 subunits, has different levels of phosphorylation on serines 408 and 409 (S408/9) between the two receptor subtypes. To understand the role S408/9 plays in the assembly of α1- and α4-containing GABA A Rs, we examined the effects of S408/9A (alanine) knock-in mutation on the subunit composition of the two receptor subtypes using LC-MS/MS and quantitative analysis. We discovered that the S408/9A mutation results in the formation of novel α1α4-containing GABA A Rs. Moreover, in S408/9A mutants, the plasma membrane expression of the α4 subunit is increased whereas its retention in the endoplasmic reticulum is reduced. These findings suggest that S408/9 play a critical role in determining the subtype-specific assembly of GABA A Rs, and thus the efficacy of neuronal inhibition.
Keywords: GABAA receptors; phosphorylation; protein purification; subunit composition; trafficking.
Copyright © 2022 Choi, Smalley, Lemons, Ren, Bope, Dengler, Davies and Moss.
Conflict of interest statement
Author SM serves as a consultant for AstraZeneca and SAGE Therapeutics, relationships that are regulated by Tufts University. Author SM holds stock in SAGE Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

