Development of the PREDS score to predict in-hospital mortality of patients with Ebola virus disease under advanced supportive care: Results from the EVISTA cohort in the Democratic Republic of the Congo
- PMID: 36263398
- PMCID: PMC9574409
- DOI: 10.1016/j.eclinm.2022.101699
Development of the PREDS score to predict in-hospital mortality of patients with Ebola virus disease under advanced supportive care: Results from the EVISTA cohort in the Democratic Republic of the Congo
Abstract
Background: As mortality remains high for patients with Ebola virus disease (EVD) despite new treatment options, the ability to level up the provided supportive care and to predict the risk of death is of major importance. This analysis of the EVISTA cohort aims to describe advanced supportive care provided to EVD patients in the Democratic Republic of the Congo (DRC) and to develop a simple risk score for predicting in-hospital death, called PREDS.
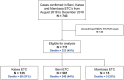
Methods: In this prospective cohort (NCT04815175), patients were recruited during the 10th EVD outbreak in the DRC across three Ebola Treatment Centers (ETCs). Demographic, clinical, biological, virological and treatment data were collected. We evaluated factors known to affect the risk of in-hospital death and applied univariate and multivariate Cox proportional-hazards analyses to derive the risk score in a training dataset. We validated the score in an internal-validation dataset, applying C-statistics as a measure of discrimination.
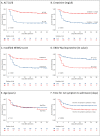
Findings: Between August 1st 2018 and December 31th 2019, 711 patients were enrolled in the study. Regarding supportive care, patients received vasopressive drug (n = 111), blood transfusion (n = 101), oxygen therapy (n = 250) and cardio-pulmonary ultrasound (n = 15). Overall, 323 (45%) patients died before day 28. Six independent prognostic factors were identified (ALT, creatinine, modified NEWS2 score, viral load, age and symptom duration). The final score range from 0 to 13 points, with a good concordance (C = 86.24%) and calibration with the Hosmer-Lemeshow test (p = 0.12).
Interpretation: The implementation of advanced supportive care is possible for EVD patients in emergency settings. PREDS is a simple, accurate tool that could help in orienting early advanced care for at-risk patients after external validation.
Funding: This study was funded by ALIMA.
Keywords: Ebola virus; In-hospital mortality; Outcome; Predictive score; Sub-Saharan Africa; Supportive care.
© 2022 The Author(s).
Conflict of interest statement
No conflict of interest is declared by any of the authors. SM is listed as the inventor on the patent application for mAb 114, US Application No.62/087, 087 (PCT Application No.PCT/US2015/060733) related to anti-Ebola virus antibodies and their use.
Figures
References
-
- 10th Ebola outbreak in the Democratic Republic of the Congo declared over; vigilance against flare-ups and support for survivors must continue.https://www.who.int/news/item/25-06-2020-10th-ebola-outbreak-in-the-demo.... Accessed 21 July 2022.
-
- Coller B-AG, Blue J, Das R, et al. Clinical development of a recombinant Ebola vaccine in the midst of an unprecedented epidemic. Vaccine. 2017;35:4465–4469. - PubMed
-
- Fischer WA, Crozier I, Bausch DG, et al. Shifting the paradigm - applying universal standards of care to ebola virus disease. N Engl J Med. 2019;380:1389–1391. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials