Cytomegalovirus reactivation after CD19 CAR T-cell therapy is clinically significant
- PMID: 36263844
- PMCID: PMC9890024
- DOI: 10.3324/haematol.2022.281719
Cytomegalovirus reactivation after CD19 CAR T-cell therapy is clinically significant
Figures
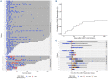
References
-
- Zuhair M, Smit GSA, Wallis G, et al. . Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034. - PubMed
-
- Wittmann Dayagi T, Sherman G, Bielorai B, et al. . Characteristics and risk factors of infections following CD28-based CD19 CAR-T cells. Leuk Lymphoma. 2021;62(7):1692-1701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

