Haemophilus influenzae Protein D antibody suppression in a multi-component vaccine formulation
- PMID: 36263849
- PMCID: PMC9714371
- DOI: 10.1002/2211-5463.13498
Haemophilus influenzae Protein D antibody suppression in a multi-component vaccine formulation
Abstract
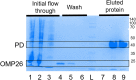
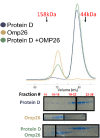
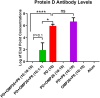
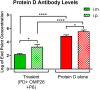
Nontypeable Haemophilus influenzae (NTHi) has emerged as a dominant mucosal pathogen causing acute otitis media (AOM) in children, acute sinusitis in children and adults, and acute exacerbations of chronic bronchitis in adults. Consequently, there is an urgent need to develop a vaccine to protect against NTHi infection. A multi-component vaccine will be desirable to avoid emergence of strains expressing modified proteins allowing vaccine escape. Protein D (PD), outer membrane protein (OMP) 26, and Protein 6 (P6) are leading protein vaccine candidates against NTHi. In pre-clinical research using mouse models, we found that recombinantly expressed PD, OMP26, and P6 induce robust antibody responses after vaccination as individual vaccines, but when PD and OMP26 were combined into a single vaccine formulation, PD antibody levels were significantly lower. We postulated that PD and OMP26 physiochemically interacted to mask PD antigenic epitopes resulting in the observed effect on antibody response. However, column chromatography and mass spectrometry analysis did not support our hypothesis. We postulated that the effect might be in vivo through the mechanism of protein vaccine immunologic antigenic competition. We found when PD and OMP26 were injected into the same leg or separate legs of mice, so that antigens were immunologically processed at the same or different regional lymph nodes, respectively, antibody levels to PD were significantly lower with same leg vaccination. Different leg vaccination produced PD antibody levels quantitatively similar to vaccination with PD alone. We conclude that mixing PD and OMP26 into a single vaccine formulation requires further formulation studies.
Keywords: Haemophilus influenzae; Protein D; acute otitis media; antibody suppression; antigenic competition; outer membrane protein 26.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
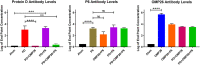


References
-
- Barkai G, Leibovitz E, Givon‐Lavi N, Dagan R. Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatr Infect Dis J. 2009;28:466–71. - PubMed
-
- Pichichero ME. Vaccine‐induced immunologic memory and pace of pathogenesis: predicting the need for boosters. Expert Rev Vaccines. 2008;7:1299–303. - PubMed
-
- Leibovitz E, Asher E, Piglansky L, Givon‐Lavi N, Satran R, Raiz S, et al. Is bilateral acute otitis media clinically different than unilateral acute otitis media? Pediatr Infect Dis J. 2007;26:589–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

