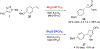Asymmetric Cyclopropanation with 4-Aryloxy-1-sulfonyl-1,2,3-triazoles: Expanding the Range of Rhodium-Stabilized Donor/Acceptor Carbenes to Systems with an Oxygen Donor Group
- PMID: 36264239
- PMCID: PMC10290286
- DOI: 10.1021/acs.joc.2c00978
Asymmetric Cyclopropanation with 4-Aryloxy-1-sulfonyl-1,2,3-triazoles: Expanding the Range of Rhodium-Stabilized Donor/Acceptor Carbenes to Systems with an Oxygen Donor Group
Abstract
Rhodium-catalyzed enantioselective synthesis of 1-phenoxycyclopropane-1-carbaldehydes by intermolecular cyclopropanation of terminal alkenes followed by imine hydrolysis is described. This methodology utilizes 4-aryloxy-1-sulfonyl-1,2,3-triazoles as the carbene precursors and the chiral dirhodium(II) tetracarboxylates Rh2(S-NTTL)4 or Rh2(S-DPCP)4 as the catalysts. These reactions are considered to proceed via rhodium-stabilized donor/acceptor carbene intermediates, and these studies demonstrate that a heteroatom donor group is compatible with an enantioselective transformation.
Conflict of interest statement
HMLD is a named inventor on a patent entitled, Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto (US 8,974,428, issued March 10, 2015). The other authors have no competing financial interests.
Figures
References
-
- For an overview of reactions of rhodium metallocarbenes, and a more detailed overview of different classes of rhodium catalysts and diazo compounds, see:
- Davies HML Finding Opportunities from Surprises and Failures. Development of Rhodium-Stabilized Donor/Acceptor Carbenes and Their Application to Catalyst-Controlled C−H Functionalization. J. Org. Chem 2019, 84, 12722–12745, and references cited therein. - PMC - PubMed
-
- For a recent review of the utility of diazo compounds as carbene precursors in organic synthesis, see:
- Dong S; Liu X; Feng X Asymmetric Catalytic Rearrangements with α-Diazocarbonyl Compounds. Acc. Chem. Res 2022, 55, 415–428. - PubMed
-
- For a general overview of reactions of N-sulfonyl-1,2,3-triazoles, see:
- Davies HML; Alford JS Reactions of Metallocarbenes Derived from Nsulfonyl-1,2,3-triazoles. Chem. Soc. Rev, 2014, 43, 5151–5162. - PubMed
-
- Alford JS; Davies HML Expanding the Scope of Donor/Acceptor Carbenes to N-Phthalimido Donor Groups: Diastereoselective Synthesis of 1-Cyclopropane α-Amino Acids. Org. Lett 2012, 14, 6020–6023. - PubMed
- Wilkerson-Hill SM; Haines BE; Musaev DG; Davies HML Synthesis of [3a,7a]-Dihydroindoles by a Tandem Arene Cyclopropanation/3,5-Sigmatropic Rearrangement Reaction. J. Org. Chem 2018, 83, 7939–7949. - PMC - PubMed
-
- For initial work demonstrating the formation of latent diazos from N-sulfonyltriazoles, see:
- Chuprakov S; Kwok SW; Zhang L; Lercher L; Fokin VV Rhodium-Catalyzed Enantioselective Cyclopropanation of Olefins with N-Sulfonyl 1,2,3-Triazoles. J. Am. Chem. Soc 2009, 131, 18034–18035. - PMC - PubMed
- Grimster N; Zhang L; Fokin VV Synthesis and Reactivity of Rhodium(II) N-Triflyl Azavinyl Carbenes. J. Am. Chem. Soc 2010, 132, 2510–2511. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources