Functional interdependence of the actin regulators CAP1 and cofilin1 in control of dendritic spine morphology
- PMID: 36264429
- PMCID: PMC9585016
- DOI: 10.1007/s00018-022-04593-8
Functional interdependence of the actin regulators CAP1 and cofilin1 in control of dendritic spine morphology
Erratum in
-
Correction: Functional interdependence of the actin regulators CAP1 and cofilin1 in control of dendritic spine morphology.Cell Mol Life Sci. 2024 Jan 18;81(1):46. doi: 10.1007/s00018-023-05039-5. Cell Mol Life Sci. 2024. PMID: 38236280 Free PMC article. No abstract available.
Abstract
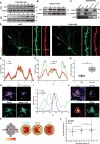
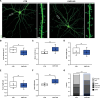
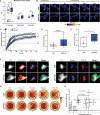
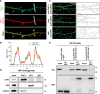
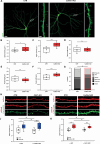
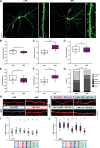
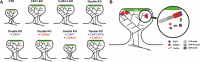
The vast majority of excitatory synapses are formed on small dendritic protrusions termed dendritic spines. Dendritic spines vary in size and density that are crucial determinants of excitatory synaptic transmission. Aberrations in spine morphogenesis can compromise brain function and have been associated with neuropsychiatric disorders. Actin filaments (F-actin) are the major structural component of dendritic spines, and therefore, actin-binding proteins (ABP) that control F-actin dis-/assembly moved into the focus as critical regulators of brain function. Studies of the past decade identified the ABP cofilin1 as a key regulator of spine morphology, synaptic transmission, and behavior, and they emphasized the necessity for a tight control of cofilin1 to ensure proper brain function. Here, we report spine enrichment of cyclase-associated protein 1 (CAP1), a conserved multidomain protein with largely unknown physiological functions. Super-resolution microscopy and live cell imaging of CAP1-deficient hippocampal neurons revealed impaired synaptic F-actin organization and dynamics associated with alterations in spine morphology. Mechanistically, we found that CAP1 cooperates with cofilin1 in spines and that its helical folded domain is relevant for this interaction. Moreover, our data proved functional interdependence of CAP1 and cofilin1 in control of spine morphology. In summary, we identified CAP1 as a novel regulator of the postsynaptic actin cytoskeleton that is essential for synaptic cofilin1 activity.
Keywords: Actin dynamics; Actin turnover; Postsynaptic density; Synaptic actin; Synaptic plasticity.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

