Impact of Ruxolitinib Cream on Work Productivity and Activity Impairment and Associated Indirect Costs in Patients with Atopic Dermatitis: Pooled Results From Two Phase III Studies
- PMID: 36264430
- PMCID: PMC9870821
- DOI: 10.1007/s40257-022-00734-8
Impact of Ruxolitinib Cream on Work Productivity and Activity Impairment and Associated Indirect Costs in Patients with Atopic Dermatitis: Pooled Results From Two Phase III Studies
Abstract
Background: Atopic dermatitis is a chronic inflammatory skin disease that can negatively impact work productivity and daily activities. Ruxolitinib cream, a Janus kinase inhibitor, demonstrated efficacy and safety in patients with atopic dermatitis in two phase III studies (TRuE-AD1 and TRuE-AD2).
Objective: This post hoc analysis sought to describe the effects of ruxolitinib cream on work productivity and activity impairment from pooled data from the phase III studies, to estimate indirect costs due to atopic dermatitis, and to estimate the incremental cost savings with ruxolitinib cream versus vehicle cream.
Methods: Patients in both studies were ≥ 12 years old with atopic dermatitis for ≥ 2 years, an Investigator's Global Assessment score of 2 or 3, and a 3-20% affected body surface area at baseline. Patients were randomized 2:2:1 to receive ruxolitinib cream (0.75% or 1.5%) or vehicle cream for 8 weeks. Patient self-reported productivity in the efficacy-evaluable population was assessed at weeks 2, 4, and 8 using the Work Productivity and Activity Impairment Questionnaire-Specific Health Problem version 2.0. Statistical significance for the two doses versus vehicle was calculated using an analysis of covariance. Work Productivity and Activity Impairment overall work impairment scores were converted to a model of costs per employed patient due to lost productivity and incremental cost savings from ruxolitinib cream treatment using a human capital approach.
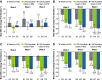
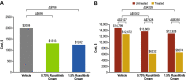
Results: Of 1249 patients enrolled (median age, 32 years; female sex, 61.7%), 1208 were included in the efficacy-evaluable population. Patients applying 0.75% or 1.5% ruxolitinib cream had significant changes in overall work impairment (- 17.9% [0.75% strength] and - 15.0% [1.5% strength] vs - 5.7% for vehicle; p < 0.0001 for both) and daily activity impairment (- 20.6% [0.75% strength] and - 21.5% [1.5% strength] vs - 10.6% for vehicle; p < 0.0001 for both). These corresponded to estimated lost productivity costs in 2021 US dollars of $1313 (0.75% strength) and $1242 (1.5% strength) versus $2008 (vehicle) over the 8-week trial period. Compared with a patient receiving vehicle, incremental annual indirect cost savings were estimated to be $5302 with 0.75% ruxolitinib cream and $4228 with 1.5% ruxolitinib cream.
Conclusions: Ruxolitinib cream therapy is associated with improved work productivity and daily activity compared with vehicle and is estimated to reduce the indirect cost burden on the patient.
Clinical trial registration: ClinicalTrials.gov identifiers: NCT03745638 (registered 19 November, 2018) and NCT03745651 (registered 19 November, 2018).
© 2022. The Author(s).
Conflict of interest statement
Lisa Bloudek, Kristen Migliaccio-Walle, and Sean D. Sullivan are employees of Curta Inc. and served as paid consultants to Incyte Corporation in connection with this study. Lawrence F. Eichenfield has served as an investigator, consultant, speaker, or data safety monitoring board member for AbbVie, Almirall, Arcutis, Asana BioSciences, Dermavant, Eli Lilly, Forte Biosciences, Galderma, Ichnos/Glenmark, Incyte Corporation, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Otsuka, Pfizer, Regeneron, and Sanofi Genzyme. Jonathan I. Silverberg has received honoraria for advisory board, speaker, and consultant services from AbbVie, Asana, Bluefin, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Incyte Corporation, Kiniksa, LEO Pharma, Menlo, Novartis, Pfizer, Realm, Regeneron, and Sanofi; and research grants for investigator services from GlaxoSmithKline and Galderma. Vijay N. Joish, Jennifer H. Lofland, and Kang Sun are employees and shareholders of Incyte Corporation. Matthias Augustin has served as a consultant, researcher, and/or has received research grants from AbbVie, ALK-Abelló, Almirall, Amgen, Bayer, Beiersdorf, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Dermira, Eli Lilly, Galderma, Genzyme, GlaxoSmithKline, Hexal, Janssen, LEO Pharma, Medac, Menlo Therapeutics, MSD, Mylan B.V., Novartis, Pfizer, Regeneron, Sandoz, Sanofi, Stallergenes, Trevi, and XenoPort. Sean D. Sullivan is an owner of VeriTech Corporation and served as a consultant to Incyte Corporation in connection with this study.
Figures
References
-
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi: 10.1016/j.jid.2018.08.028. - DOI - PubMed

