Loss of function variants in DNAJB4 cause a myopathy with early respiratory failure
- PMID: 36264506
- PMCID: PMC9812937
- DOI: 10.1007/s00401-022-02510-8
Loss of function variants in DNAJB4 cause a myopathy with early respiratory failure
Abstract
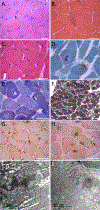
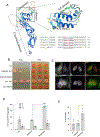
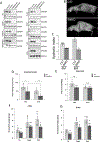
DNAJ/HSP40 co-chaperones are integral to the chaperone network, bind client proteins and recruit them to HSP70 for folding. We performed exome sequencing on patients with a presumed hereditary muscle disease and no genetic diagnosis. This identified four individuals from three unrelated families carrying an unreported homozygous stop gain (c.856A > T; p.Lys286Ter), or homozygous missense variants (c.74G > A; p.Arg25Gln and c.785 T > C; p.Leu262Ser) in DNAJB4. Affected patients presented with axial rigidity and early respiratory failure requiring ventilator support between the 1st and 4th decade of life. Selective involvement of the semitendinosus and biceps femoris muscles was seen on MRI scans of the thigh. On biopsy, muscle was myopathic with angular fibers, protein inclusions and occasional rimmed vacuoles. DNAJB4 normally localizes to the Z-disc and was absent from muscle and fibroblasts of affected patients supporting a loss of function. Functional studies confirmed that the p.Lys286Ter and p.Leu262Ser mutant proteins are rapidly degraded in cells. In contrast, the p.Arg25Gln mutant protein is stable but failed to complement for DNAJB function in yeast, disaggregate client proteins or protect from heat shock-induced cell death consistent with its loss of function. DNAJB4 knockout mice had muscle weakness and fiber atrophy with prominent diaphragm involvement and kyphosis. DNAJB4 knockout muscle and myotubes had myofibrillar disorganization and accumulated Z-disc proteins and protein chaperones. These data demonstrate a novel chaperonopathy associated with DNAJB4 causing a myopathy with early respiratory failure. DNAJB4 loss of function variants may lead to the accumulation of DNAJB4 client proteins resulting in muscle dysfunction and degeneration.
Keywords: Chaperone; Congenital myopathy; Myofibrillar myopathy; Myopathy; Protein aggregation.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

