Association of Long-term Outcomes With Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial
- PMID: 36264568
- PMCID: PMC9585461
- DOI: 10.1001/jamaoncol.2022.5049
Association of Long-term Outcomes With Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial
Abstract
Importance: Long-term outcomes of radiotherapy are important in understanding the risks and benefits of therapies for patients with brain metastases.
Objective: To determine how the use of postoperative whole-brain radiotherapy (WBRT) or stereotactic radiosurgery (SRS) is associated with quality of life (QOL), cognitive function, and intracranial tumor control in long-term survivors with 1 to 4 brain metastases.
Design, setting, and participants: This secondary analysis of a randomized phase 3 clinical trial included 48 institutions in the US and Canada. Adult patients with 1 resected brain metastases but limited to those with 1 to 4 brain metastasis were eligible. Unresected metastases were treated with SRS. Long-term survivors were defined as evaluable patients who lived longer than 1 year from randomization. Patients were recruited between July 2011 and December 2015, and data were first analyzed in February 2017. For the present study, intracranial tumor control, cognitive deterioration, QOL, and cognitive outcomes were measured in evaluable patients who were alive at 12 months from randomization and reanalyzed in June 2017.
Interventions: Stereotactic radiosurgery or WBRT.
Main outcomes and measures: Intracranial tumor control, toxic effects, cognitive deterioration, and QOL.
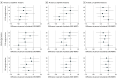
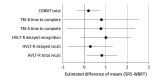
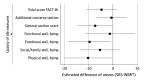
Results: Fifty-four patients (27 SRS arm, 27 WBRT arm; female to male ratio, 65% vs 35%) were included for analysis with a median follow-up of 23.8 months. Cognitive deterioration was less frequent with SRS (37%-60%) compared with WBRT (75%-91%) at all time points. More patients declined by 2 or more standard deviations (SDs) in 1 or more cognitive tests for WBRT compared with SRS at 3, 6, and 9 months (70% vs 22%, 46% vs 19%, and 50% vs 20%, respectively). A 2 SD decline in at least 2 cognitive tests was associated with worse 12-month QOL in emotional well-being, functional well-being, general, additional concerns, and total scores. Overall QOL and functional independence favored SRS alone for categorical change at all time points. Total intracranial control for SRS alone vs WBRT at 12 months was 40.7% vs 81.5% (difference, -40.7; 95% CI, -68.1% to -13.4%), respectively. Data were first analyzed in February 2017.
Conclusions and relevance: The use of SRS alone compared with WBRT resulted in less cognitive deterioration among long-term survivors. The association of late cognitive deterioration with WBRT was clinically meaningful. A significant decline in cognition (2 SD) was associated with overall QOL. However, intracranial tumor control was improved with WBRT. This study provides detailed insight into cognitive function over time in this patient population.
Trial registration: ClinicalTrials.gov Identifier: NCT01372774; ALLIANCE/CCTG: N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group).
Conflict of interest statement
Figures
References
-
- Aoyama H, Tago M, Shirato H; Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators . Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464. doi: 10.1001/jamaoncol.2015.1145 - DOI - PubMed
-
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672. doi: 10.1016/S0140-6736(04)16250-8 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

