Ocular Monkeypox - United States, July-September 2022
- PMID: 36264836
- PMCID: PMC9590292
- DOI: 10.15585/mmwr.mm7142e1
Ocular Monkeypox - United States, July-September 2022
Abstract
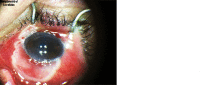
As of October 11, 2022, a total of 26,577 monkeypox cases had been reported in the United States.* Although most cases of monkeypox are self-limited, lesions that involve anatomically vulnerable sites can cause complications. Ocular monkeypox can occur when Monkeypox virus (MPXV) is introduced into the eye (e.g., from autoinoculation), potentially causing conjunctivitis, blepharitis, keratitis, and loss of vision (1). This report describes five patients who acquired ocular monkeypox during July-September 2022. All patients received treatment with tecovirimat (Tpoxx)†; four also received topical trifluridine (Viroptic).§ Two patients had HIV-associated immunocompromise and experienced delays between clinical presentation with monkeypox and initiation of monkeypox-directed treatment. Four patients were hospitalized, and one experienced marked vision impairment. To decrease the risk for autoinoculation, persons with monkeypox should be advised to practice hand hygiene and to avoid touching their eyes, which includes refraining from using contact lenses (2). Health care providers and public health practitioners should be aware that ocular monkeypox, although rare, is a sight-threatening condition. Patients with signs and symptoms compatible with ocular monkeypox should be considered for urgent ophthalmologic evaluation and initiation of monkeypox-directed treatment. Public health officials should be promptly notified of cases of ocular monkeypox. Increased clinician awareness of ocular monkeypox and of approaches to prevention, diagnosis, and treatment might reduce associated morbidity.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. James Chodosh is a consultant to the Food and Drug Administration, where he chairs an advisory committee for new ophthalmic medications. He also receives grant support from the National Institutes of Health (NIH) to study adenovirus keratitis. Caitlin M. Dugdale reports institutional support from the National Institute for Child Health and Human Development, NIH; Harvard University Center for AIDS Research; the Massachusetts General Hospital Executive Committee on Research; the International AIDS Vaccine Initiative; and the International Maternal, Pediatric, Adolescent AIDS Clinical Trials (IMPAACT) Network, NIH. Aaron R. Kaufman reports support by a Heed Fellowship awarded by the Heed Ophthalmic Foundation. Roberto Pineda II reports royalties from Elsevier and consulting fees from Sanofi-Genzyme. No other potential conflicts of interest were disclosed.
Figures

References
-
- CDC. Monkeypox. Isolation and infection control at home. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. Accessed October 12, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-home...
-
- CDC. Emergency preparedness and response. Severe manifestations of monkeypox among people who are immunocompromised due to HIV or other conditions. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. Accessed October 6, 2022. https://emergency.cdc.gov/han/2022/han00475.asp
-
- CDC. Monkeypox. Technical report 3: multi-national monkeypox outbreak, United States, 2022. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. Accessed October 12, 2022. https://www.cdc.gov/poxvirus/monkeypox/cases-data/technical-report/repor...
-
- Hughes C, McCollum A, Pukuta E, et al. Ocular complications associated with acute monkeypox virus infection, DRC. Int J Infect Dis 2014;21:276–7 10.1016/j.ijid.2014.03.994 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

