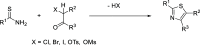Synthesis of a Thiazole Library via an Iridium-Catalyzed Sulfur Ylide Insertion Reaction
- PMID: 36265082
- PMCID: PMC9641659
- DOI: 10.1021/acs.orglett.2c02996
Synthesis of a Thiazole Library via an Iridium-Catalyzed Sulfur Ylide Insertion Reaction
Abstract
A library of thiazoles and selenothiazoles were synthesized via Ir-catalyzed ylide insertion chemistry. This process is a functional group, particularly heterocycle-substituent tolerant. This was applied to the synthesis of fanetizole, an anti-inflammatory drug, and a thiazole-containing drug fragment that binds to the peptidyl-tRNA hydrolase (Pth) in Neisseria gonorrheae bacteria.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- de Souza M. V. N. Synthesis and Biological Activity of Natural Thiazoles: An Important Class of Heterocyclic Compounds. J. Sulfur Chem. 2005, 26 (4–5), 429–449. 10.1080/17415990500322792. - DOI
-
- Ripain I. H. A.; Ngah N. A Brief Review on the Thiazole Derivatives: Synthesis Methods and Biological Activities. Malaysian J. Anal. Sci. 2021, 25 (2), 257–267.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources