Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial
- PMID: 36265087
- PMCID: PMC10651777
- DOI: 10.1182/blood.2022016362
Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial
Abstract
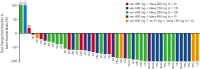
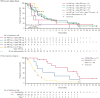
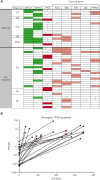
This phase 1b trial (NCT02670044) evaluated venetoclax-idasanutlin in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML) ineligible for cytotoxic chemotherapy. Two-dimensional dose escalation (DE, n = 50) was performed for venetoclax daily with idasanutlin on days 1 to 5 in 28-day cycles, followed by dosing schedule optimization (n = 6) to evaluate reduced venetoclax schedules (21-/14-day dosing). Common adverse events (occurring in ≥40% of patients) included diarrhea (87.3% of patients), nausea (74.5%), vomiting (52.7%), hypokalemia (50.9%), and febrile neutropenia (45.5%). During DE, across all doses, composite complete remission (CRc; CR + CR with incomplete blood count recovery + CR with incomplete platelet count recovery) rate was 26.0% and morphologic leukemia-free state (MLFS) rate was 12%. For anticipated recommended phase 2 doses (venetoclax 600 mg + idasanutlin 150 mg; venetoclax 600 mg + idasanutlin 200 mg), the combined CRc rate was 34.3% and the MLFS rate was 14.3%. Pretreatment IDH1/2 and RUNX1 mutations were associated with higher CRc rates (50.0% and 45.0%, respectively). CRc rate in patients with TP53 mutations was 20.0%, with responses noted among those with co-occurring IDH and RUNX1 mutations. In 12 out of 36 evaluable patients, 25 emergent TP53 mutations were observed; 22 were present at baseline with low TP53 variant allele frequency (median 0.0095% [range, 0.0006-0.4]). Venetoclax-idasanutlin showed manageable safety and encouraging efficacy in unfit patients with R/R AML. IDH1/2 and RUNX1 mutations were associated with venetoclax-idasanutlin sensitivity, even in some patients with co-occurring TP53 mutations; most emergent TP53 clones were preexisting. Our findings will aid ongoing/future trials of BCL-2/MDM2 inhibitor combinations. This trial was registered at www.clinicaltrials.gov as #NCT02670044.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: N.G.D. has received research funding from AbbVie and Genentech. M.D., M.O. J.W., and W.H. are employees of Genentech and may hold Roche stock or stock options. J.S.G. has received research funding (for trials) from AbbVie, Genentech, Pfizer, Prelude, and AstraZeneca; and has served on advisory board for AbbVie, Astellas, and Takeda. B.A.J. is a consultant/advisor for AbbVie, BMS, Celgene, Genentech, Gilead, GlycoMimetics, Jazz, Pfizer, Servier, Takeda, Tolero, and Treadwell; has received travel reimbursement from AbbVie; and research funding to his institution from 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, BMS, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma Therapeutics, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz, Loxo, LP Therapeutics, Pfizer, Pharmacyclics, Sigma-Tau and Treadwell. K.W.L.Y. is a consultant for Astex, BMS/Celgene, F. Hoffmann-La Roche, Novartis, Otsuka, Paladin, Pfizer, Shattuck Labs, Taiho, and Takeda; has received honorarium from AbbVie and Novartis; and has received research funding from Astex, Forma Therapeutics, F. Hoffmann-La Roche, Genentech, Geron, Janssen, Jazz, MedImmune, Novartis, Onconova, and Tolero. K.R.K. received honoraria from Janssen, Novartis, Celgene, Epizyme, Pharmacyclics, Karyopharm, GSK, Bristol-Myers Squibb, Seattle Genetics, and Gilead, consulting fees from Sanofi, AstraZeneca, Sanofi-Aventis, Denovo Biopharma and Amgen and received research funding from Takeda and Oncolytics Biotech Inc. S.A. reports nonfinancial support from Roche/Genentech during the conduct of the study; and has received personal fees from Roche Canada, Pfizer, BMS, Paladin, and Lundbeck outside the submitted work. G.J.R. consultancy AbbVie, Agios, Amgen, Amphivena, Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche, Sunesis, and Jazz; and has received research funding from Cellectis. D.A.P. serves on a data safety monitoring board for GlycoMimetics and Aptevo; has received research funding from AbbVie, BMS, Teva and Karyopharm; and is an advisory board member or consultant for Novartis, AbbVie, BeiGene, BerGenBio, Arcellx, Jazz, Syros, BMS, Genentech, ImmunoGen, AstraZeneca, Kura, Ryvu, Magenta, and Qihan Zentalis. J.M.B. consultancy AbbVie, BMS, Astex, Pfizer, Astellas, Taiho, and Jazz. R.L.O. has received research support for trials from Astellas, Pfizer, Genentech, Daiichi Sankyo, and Cellectis; and consulting from AbbVie, Astellas, and Actinium. G.M. is a consultant for AbbVie, Celgene, Roche, Janssen, Astellas, Pfizer, and Incyte. B.L.P. has received research funding from Ambit Biosciences, Genentech, F. Hoffmann-La Roche, Jazz, Novartis, Pfizer, and Rafael Pharmaceuticals; and is a consultant for Rafael Pharmaceuticals. W-J.H. is a former employee of Genentech and may hold Roche stock; is a former employee of Imago BioSciences and may hold stock; and is a current employee of Prelude Therapeutics. M.Y.K. is a consultant for AbbVie, Genentech, and F. Hoffmann-La Roche; is an advisory board member for F. Hoffmann-La Roche; holds shares from Reata; has received honoraria from Amgen, AbbVie, and Genentech; and has received research funding from AbbVie, Genentech, Eli Lilly, Cellectis, Calithera, Stemline, Threshold, Flexus Biosciences, Novartis, Ablynx, and Agios. M.A. consulted for Amgen, AstraZeneca, Daiichi Sankyo, Syndax, GlycoMimetics, Oncoceutics, and Aptose; has received research funding from F. Hoffmann-La Roche, AstraZeneca, Amgen, Daiichi Sankyo, Jazz, GlycoMimetics; and holds stock from Reata, Oncoceutics/Chimerix and Aptose. The remaining authors declare no competing financial interests.
Figures

Comment in
-
MDM2 and BCL-2: to p53 or not to p53?Blood. 2023 Mar 16;141(11):1237-1238. doi: 10.1182/blood.2022018739. Blood. 2023. PMID: 36929437 No abstract available.
References
-
- Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919–1926. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

