Chemoselective Solution- and Solid-Phase Synthesis of Disulfide-Linked Glycopeptides
- PMID: 36265181
- PMCID: PMC9638999
- DOI: 10.1021/acs.joc.2c01651
Chemoselective Solution- and Solid-Phase Synthesis of Disulfide-Linked Glycopeptides
Abstract
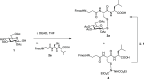
Glycosylation of peptides and proteins is a widely employed strategy to mimic important post-translational modifications or to modulate the physicochemical properties of peptides to enhance their delivery. Furthermore, glycosylation via a sulfur atom imparts increased chemical and metabolic stability to the resulting glycoconjugates. Herein, we report a simple and chemoselective procedure to prepare disulfide-linked glycopeptides. Acetate-protected glycosylsulfenyl hydrazines are shown to be highly reactive with the thiol group of cysteine residues within peptides, both in solution and as part of conventional solid-phase peptide synthesis protocols. The efficiency of this glycosylation methodology with unprotected carbohydrates is also demonstrated, which avoids the need for deprotection steps and further extends its utility, with disulfide-linked glycopeptides produced in excellent yields. Given the importance of glycosylated peptides in structural glycobiology, pharmacology, and therapeutics, the methodology outlined provides easy access to disulfide-linked glycopeptides as molecules with multiple biological applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Chemical synthesis of the S-linked glycopeptide, sublancin.J Pept Sci. 2011 Dec;17(12):818-21. doi: 10.1002/psc.1406. Epub 2011 Sep 29. J Pept Sci. 2011. PMID: 21957066
-
Glycopeptide and glycoprotein synthesis involving unprotected carbohydrate building blocks.Med Res Rev. 2005 Nov;25(6):655-78. doi: 10.1002/med.20033. Med Res Rev. 2005. PMID: 15895471 Review.
-
Solid phase synthesis of glycopeptides using Shoda's activation of unprotected carbohydrates.Chem Commun (Camb). 2013 Sep 7;49(69):7608-10. doi: 10.1039/c3cc43458c. Epub 2013 Jul 24. Chem Commun (Camb). 2013. PMID: 23882465
-
Of Thiols and Disulfides: Methods for Chemoselective Formation of Asymmetric Disulfides in Synthetic Peptides and Polymers.Chemistry. 2018 Aug 22;24(47):12131-12142. doi: 10.1002/chem.201800681. Epub 2018 Jul 6. Chemistry. 2018. PMID: 29645294
-
Optimization of physicochemical and pharmacological properties of peptide drugs by glycosylation.Methods Mol Biol. 2013;1081:107-36. doi: 10.1007/978-1-62703-652-8_8. Methods Mol Biol. 2013. PMID: 24014437 Review.
Cited by
-
Exploring the Chemistry and Applications of Thio-, Seleno-, and Tellurosugars.Molecules. 2025 May 5;30(9):2053. doi: 10.3390/molecules30092053. Molecules. 2025. PMID: 40363858 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

