Allergy Workup in the Diagnosis of COVID-19 Vaccines-Induced Hypersensitivity Reactions and Its Impact on Vaccination
- PMID: 36265449
- PMCID: PMC9747735
- DOI: 10.1159/000526764
Allergy Workup in the Diagnosis of COVID-19 Vaccines-Induced Hypersensitivity Reactions and Its Impact on Vaccination
Abstract
Introduction: Immediate and delayed hypersensitivity reactions (HSR) to COVID-19 vaccines are rare adverse events that need to be prevented, diagnosed, and managed in order to guarantee adherence to the vaccination campaign. The aims of our study were to stratify the risk of HSR to COVID-19 vaccines and propose alternative strategies to complete the vaccination.
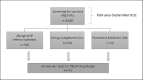
Methods: 1,640 subjects were screened for vaccinal eligibility, according to national and international recommendations. Among them, we enrolled for allergy workup 152 subjects, 43 with HSR to COVID-19 vaccines and 109 at high risk of HSR to the first dose. In vivo skin tests with drugs and/or vaccines containing PEG/polysorbates were performed in all of them, using skin prick test and, when negative, intradermal tests. In a subgroup of patients resulted negative to the in vivo skin tests, the programmed dose of COVID-19 vaccine (Pfizer/BioNTech) was administered in graded doses regimen, and detection of neutralizing anti-spike antibodies was performed in these patients after 4 weeks from the vaccination, using the SPIA method.
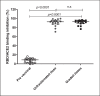
Results: Skin tests for PEG/polysorbates resulted positive in only 3% (5/152) of patients, including 2 with previous HSR to COVID-19 vaccines and 3 at high risk of HSR to the first dose. Among the 147 patients with negative skin tests, 97% (143/147) were eligible for vaccination and 87% (124/143) of them received safely the programmed COVID-19 vaccine dose. Administration of graded doses of Pfizer/BioNTech vaccine were well tolerated in 17 out of 18 patients evaluated; only 1 developed an HSR during the vaccination, less severe than the previous one, and all developed neutralizing anti-spike antibodies after 4 weeks with values comparable to those subjects who received the vaccine in unfractionated dose.
Conclusion: On the whole, the usefulness of the skin tests for PEG/polysorbates seems limited in the diagnosis of HSR to COVID-19 vaccines. Graded doses regimen (Pfizer/BioNTech) is a safe and effective alternative strategy to complete the vaccinal course.
Keywords: Allergy; COVID-19; Polyethylene glycol; Polysorbates; Skin test; Vaccine.
© 2022 S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Comment on
-
mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach.J Allergy Clin Immunol Pract. 2021 Apr;9(4):1423-1437. doi: 10.1016/j.jaip.2020.12.047. Epub 2020 Dec 31. J Allergy Clin Immunol Pract. 2021. PMID: 33388478 Free PMC article. Review.
References
-
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2 an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397((10269)):99–111. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

