Neoadjuvant immunotherapy, chemotherapy, and combination therapy in muscle-invasive bladder cancer: A multi-center real-world retrospective study
- PMID: 36265483
- PMCID: PMC9729796
- DOI: 10.1016/j.xcrm.2022.100785
Neoadjuvant immunotherapy, chemotherapy, and combination therapy in muscle-invasive bladder cancer: A multi-center real-world retrospective study
Abstract
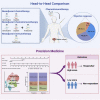
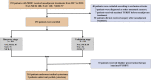
To parallelly compare the efficacy of neoadjuvant immunotherapy (tislelizumab), neoadjuvant chemotherapy (gemcitabine and cisplatin), and neoadjuvant combination therapy (tislelizumab + GC) in patients with muscle-invasive bladder cancer (MIBC) and explore the efficacy predictors, we perform a multi-center, real-world cohort study that enrolls 253 patients treated with neoadjuvant treatments (combination therapy: 98, chemotherapy: 107, and immunotherapy: 48) from 15 tertiary hospitals. We demonstrate that neoadjuvant combination therapy achieves the highest complete response rate and pathological downstaging rate compared with neoadjuvant immunotherapy or chemotherapy. We develop and validate an efficacy prediction model consisting of pretreatment clinical characteristics, which can pinpoint candidates to receive neoadjuvant combination therapy. We also preliminarily reveal that patients who achieve pathological complete response after neoadjuvant treatments plus maximal transurethral resection of the bladder tumor may be safe to receive bladder preservation therapy. Overall, this study highlights the benefit of neoadjuvant combination therapy based on tislelizumab for MIBC.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no competing interests.
Figures



References
-
- Griffiths G., Hall R., Sylvester R., Raghavan D., Parmar M.K. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 2011;29:2171–2177. doi: 10.1200/jco.2010.32.3139. - DOI - PMC - PubMed
-
- Patel H.D., Patel S.H., Blanco-Martinez E., Kuzbel J., Chen V.S., Druck A., Koehne E.L., Patel P.M., Doshi C.P., Hahn N.M., et al. Four versus 3 cycles of neoadjuvant chemotherapy for muscle-invasive bladder cancer: implications for pathological response and survival. J. Urol. 2022;207:77–85. doi: 10.1097/ju.0000000000002189. - DOI - PubMed
-
- D'Andrea D., Black P.C., Zargar H., Dinney C.P., Soria F., Cookson M.S., Montgomery J.S., Kassouf W., Dall'Era M.A., Sridhar S.S., et al. Identifying the optimal number of neoadjuvant chemotherapy cycles in patients with muscle invasive bladder cancer. J. Urol. 2022;207:70–76. doi: 10.1097/ju.0000000000002190. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

