Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice
- PMID: 36265510
- PMCID: PMC11752949
- DOI: 10.1038/s41591-022-02092-8
Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice
Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants in the Omicron lineage has resulted in diminished Coronavirus Disease 2019 (COVID-19) vaccine efficacy and persistent transmission. In this study, we evaluated the immunogenicity and protective efficacy of two, recently authorized, bivalent COVID-19 vaccines that contain two mRNAs encoding Wuhan-1 and either BA.1 (mRNA-1273.214) or BA.4/5 (mRNA-1273.222) spike proteins. As a primary two-dose immunization series in mice, both bivalent vaccines induced greater neutralizing antibody responses against Omicron variants than the parental, monovalent mRNA-1273 vaccine. When administered to mice as a booster at 7 months after the primary vaccination series with mRNA-1273, the bivalent vaccines induced broadly neutralizing antibody responses. Whereas most anti-Omicron receptor binding domain antibodies in serum induced by mRNA-1273, mRNA-1273.214 and mRNA-1273.222 boosters cross-reacted with the antecedent Wuhan-1 spike antigen, the mRNA-1273.214 and mRNA-1273.222 bivalent vaccine boosters also induced unique BA.1-specific and BA.4/5-specific responses, respectively. Although boosting with parental or bivalent mRNA vaccines substantially improved protection against BA.5 compared to mice receiving two vaccine doses, the levels of infection, inflammation and pathology in the lung were lowest in animals administered the bivalent mRNA vaccines. Thus, boosting with bivalent Omicron-based mRNA-1273.214 or mRNA-1273.222 vaccines enhances immunogenicity and confers protection in mice against a currently circulating SARS-CoV-2 strain.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures

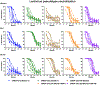

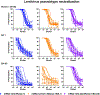






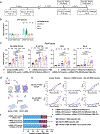

Update of
-
Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant.bioRxiv [Preprint]. 2022 Sep 13:2022.09.12.507614. doi: 10.1101/2022.09.12.507614. bioRxiv. 2022. Update in: Nat Med. 2023 Jan;29(1):247-257. doi: 10.1038/s41591-022-02092-8. PMID: 36263060 Free PMC article. Updated. Preprint.
References
MAIN TEXT REFERENCES
-
- Qi H, Liu B, Wang X & Zhang L The humoral response and antibodies against SARS-CoV-2 infection. Nat Immunol 23, 1008–1020 (2022). - PubMed
METHODS REFERENCES
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

