Myogenic tissue nanotransfection improves muscle torque recovery following volumetric muscle loss
- PMID: 36266362
- PMCID: PMC9585072
- DOI: 10.1038/s41536-022-00259-y
Myogenic tissue nanotransfection improves muscle torque recovery following volumetric muscle loss
Abstract
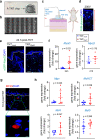
This work rests on our non-viral tissue nanotransfection (TNT) platform to deliver MyoD (TNTMyoD) to injured tissue in vivo. TNTMyoD was performed on skin and successfully induced expression of myogenic factors. TNTMyoD was then used as a therapy 7 days following volumetric muscle loss (VML) of rat tibialis anterior and rescued muscle function. TNTMyoD is promising as VML intervention.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

