Neurotensin neurons in the extended amygdala control dietary choice and energy homeostasis
- PMID: 36266470
- PMCID: PMC9682790
- DOI: 10.1038/s41593-022-01178-3
Neurotensin neurons in the extended amygdala control dietary choice and energy homeostasis
Abstract
Obesity is a global pandemic that is causally linked to many life-threatening diseases. Apart from some rare genetic conditions, the biological drivers of overeating and reduced activity are unclear. Here, we show that neurotensin-expressing neurons in the mouse interstitial nucleus of the posterior limb of the anterior commissure (IPAC), a nucleus of the central extended amygdala, encode dietary preference for unhealthy energy-dense foods. Optogenetic activation of IPACNts neurons promotes obesogenic behaviors, such as hedonic eating, and modulates food preference. Conversely, acute inhibition of IPACNts neurons reduces feeding and decreases hedonic eating. Chronic inactivation of IPACNts neurons recapitulates these effects, reduces preference for sweet, non-caloric tastants and, furthermore, enhances locomotion and energy expenditure; as a result, mice display long-term weight loss and improved metabolic health and are protected from obesity. Thus, the activity of a single neuronal population bidirectionally regulates energy homeostasis. Our findings could lead to new therapeutic strategies to prevent and treat obesity.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures
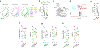




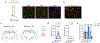




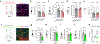


References
-
- Bluher M Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15, 288–298 (2019). - PubMed
Methods-only References
-
- Gamba OFM BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330 (2016).
-
- Mehlem A, Hagberg CE, Muhl L, Eriksson U & Falkevall A Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 8, 1149–1154 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

