Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death
- PMID: 36266697
- PMCID: PMC9583051
- DOI: 10.1186/s12916-022-02606-8
Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death
Abstract
Background: Limited data are available on the effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines in real-world use-especially against Omicron variants in SARS-CoV-2 infection-naïve population.
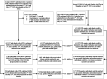
Methods: A matched case-control study was conducted among people aged ≥ 3 years between 2 December 2021 and 13 May 2022. Cases were SARS-CoV-2-infected individuals, individuals with severe/critical COVID-19, or COVID-19-related deaths. Controls were selected from consecutively test-negative individuals at the same time as cases were diagnosed and were exact-matched on year-of-age, gender, birthplace, illness onset date, and residential district in ratios of 1:1 with infected individuals and 4:1 with severe/critical COVID-19 and COVID-19-related death. Additionally, two subsets were constructed to analyze separate vaccine effectiveness (VE) of inactivated vaccines (subset 1) and Ad5-vectored vaccine (subset 2) against each of the three outcomes.
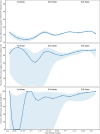
Results: Our study included 612,597 documented SARS-CoV-2 infections, among which 1485 progressed to severe or critical illness and 568 died. Administering COVID-19 vaccines provided limited protection against SARS-CoV-2 infection across all age groups (overall VE: 16.0%, 95% CI: 15.1-17.0%) but high protection against severe/critical illness (88.6%, 85.8-90.8%) and COVID-19-related death (91.6%, 86.8-94.6%). In subset 1, inactivated vaccine showed 16.3% (15.4-17.2%) effective against infection, 88.6% (85.8-90.9%) effective against severe/critical COVIID-19, and 91.7% (86.9-94.7%) against COVID-19 death. Booster vaccination with inactivated vaccines enhanced protection against severe COVID-19 (92.7%, 90.1-94.6%) and COVID-19 death (95.9%, 91.4-98.1%). Inactivated VE against infection began to wane 12 weeks after the last dose, but two and three doses sustained high protection levels (> 80%) against severe/critical illness and death, while subset 2 showed Ad5-vectored vaccine was 13.2% (10.9-15.5%) effective against infection and 77.9% (15.6-94.2%) effective against severe/critical COVIID-19.
Conclusions: Our real-world study found high and durable two- and three-dose inactivated VE against Omicron-associated severe/critical illness and death across all age groups, but lower effectiveness against Omicron infection, which reinforces the critical importance of full-series vaccination and timely booster dose administration for all eligible individuals.
Keywords: COVID-19; Case-control study; Inactivated vaccine; Vaccine effectiveness.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- World Health Organization . Statement on Omicron sublineage BA. 2. 2022.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

