Halometabolites isolated from the marine-derived fungi with potent pharmacological activities
- PMID: 36267169
- PMCID: PMC9576957
- DOI: 10.3389/fmicb.2022.1038487
Halometabolites isolated from the marine-derived fungi with potent pharmacological activities
Abstract
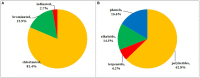
Halometabolites, usually produced in marine environment, are an important group of natural halogenated compounds with rich biological functionality and drugability and thus play a crucial role in pharmaceutical and/or agricultural applications. In the exploration of novel halometabolites from marine microorganisms, the growing number of halogenated compounds makes it necessary to fully present these metabolites with diverse structures and considerable bioactivities. This review particularly focuses on the chemodiversity and bioactivities of halometabolites from marine-derived fungi. As a result, a total of 145 naturally halogenated compounds, including 118 chlorinated, 23 brominated, and four iodinated compounds, were isolated from 17 genera of marine-derived fungi. Interestingly, many of halometabolites, especially for the brominated and iodinated compounds, are generated by the substitution of bromide and iodide ions for the chloride ion in cultivation process. In addition, these compounds possess diverse structural types, which are classified into polyketides (62.7%), phenols (16.6%), alkaloids (14.5%), and terpenoids (6.2%). Their cytotoxic, antibacterial, and anti-inflammatory activities indicate the high potential of these halogenated compounds as lead compounds for drug discovery.
Keywords: biological activities; chemical diversity; halometabolites; marine fungi; natural products.
Copyright © 2022 Chen, Xu, Liu, Zhang and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











References
-
- Chen S., Liu Z., Chen Y., Tan H., Liu H., Zhang W. (2021). Tersaphilones A-E, cytotoxic chlorinated azaphilones from the deep-sea-derived fungus Phomopsis tersa FS441. Tetrahedron 78:131806. doi: 10.1016/j.tet.2020.131806 - DOI
-
- Chen M., Zheng Y. Y., Chen Z. Q., Shen N. X., Shen L., Zhang F. M., et al. . (2019). NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 82, 368–374. doi: 10.1021/acs.jnatprod.8b00930, PMID: - DOI - PubMed
-
- Elsebai M. F., Ghabbour H. A. (2016). Isocoumarin derivatives from the marine-derived fungus Phoma sp. 135. Tetrahedron Lett. 57, 354–356. doi: 10.1016/j.tetlet.2015.12.024 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

