Severe Bradycardia Leading to Hemodynamic Instability Associated with Remdesivir Use in a Patient with COVID-19 Pneumonia
- PMID: 36267193
- PMCID: PMC9578912
- DOI: 10.1155/2022/8807957
Severe Bradycardia Leading to Hemodynamic Instability Associated with Remdesivir Use in a Patient with COVID-19 Pneumonia
Abstract
Remdesivir (RDV) is an approved treatment for hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. There is limited literature on the cardiac adverse effects of RDV. We report a case of a patient who developed hemodynamically unstable bradycardia after the initiation of RDV that resolved after discontinuing RDV.
Copyright © 2022 Bhargavi Donepudi et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures
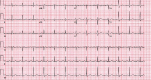

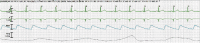

References
-
- Ader F., Bouscambert-Duchamp M., Hites M., et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. The Lancet Infectious Diseases . 2022;22(2):209–221. doi: 10.1016/S1473-3099(21)00485-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

