Design and evaluation of rufinamide nanocrystals loaded thermoresponsive nasal in situ gelling system for improved drug distribution to brain
- PMID: 36267292
- PMCID: PMC9577085
- DOI: 10.3389/fphar.2022.943772
Design and evaluation of rufinamide nanocrystals loaded thermoresponsive nasal in situ gelling system for improved drug distribution to brain
Abstract
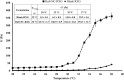
Rufinamide (Rufi) is an antiepileptic drug used to manage Lennox-Gastaut Syndrome and partial seizures. The oral bioavailability of Rufi is less due to its poor solubility and low dissolution rate in the gastrointestinal fluids. This results in less amount of drug reaching the brain following the oral administration of drug. Oral formulations of Rufi are prescribed at a high dose and dosing frequency to increase its distribution to the brain. A Rufi loaded thermoresponsive nasal in situ gel which showed significantly high brain concentrations compared to aqueous suspension of Rufi administered through nasal route was developed by our research group and published. In the current work, we have formulated nanocrystals of Rufi and suspended them in a xyloglucan based thermoresponsive gel to improve the nose-to-brain distribution. The particle size, polydispersity index, and yield (%) of the optimized Rufi nanocrystals were 261.2 ± 2.1 nm, 0.28 ± 0.08, and 89.6 ± 2.0 respectively. The narrow PDI indicates that the manufacturing process is reproducible and reliable. Higher % yield suggested that the method of preparation is efficient. The sol-to-gel transition of in situ gel loaded with Rufi nanocrystals was at 32°C which suggested that the formulation transforms into gel at nasal epithelial temperatures. The nasal pharmacokinetic studies showed that Rufi nanocrystals loaded in situ gel produced higher concentration of the drug in brain (higher brain Cmax) and maintained the drug concentrations for longer duration (higher mean residence time) compared to aqueous suspension of Rufi nanocrystals as well aqueous suspension of Rufi and Rufi loaded in situ gel, reported previously. Nanometric size of the Rufi nanocrystals combined with the in situ gelling properties helped the optimized formulation achieve higher brain distribution and also sustain the drug concentrations in brain for longer duration compared to any of the formulations studied by our research group.
Keywords: nanocrystals; nose-to-brain delivery; pharmacokinetics; rufinamide; thermoresponsive gel.
Copyright © 2022 Dalvi, Ravi and Uppuluri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Dalvi A. V., Ravi P. R., Uppuluri C. T., Mahajan R. R., Katke S. V., Deshpande V. S. (2021). Thermosensitive nasal in situ gelling systems of rufinamide formulated using modified tamarind seed xyloglucan for direct nose-to-brain delivery: Design, physical characterization, and in vivo evaluation. J. Pharm. Investig. 51 (2), 199–211. 10.1007/s40005-020-00505-9 - DOI
-
- Dalvi S. V., Dave R. N. (2009). Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind. Eng. Chem. Res. 48 (16), 7581–7593. 10.1021/ie900248f - DOI
-
- Eisai Inc. (2015). Eisai announces FDA approval of Banzel® (rufinamide) for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome in pediatric patients ages 1-4, Eisai USA. Available at https://eisai.mediaroom.com/2015-02-13-Eisai-Announces-FDA-Approval-of-B... (Accessed September 11, 2022).
-
- Emad N. A., Ahmed B., Alhalmi A., Alzobaidi N., Al-Kubati S. S. (2021). Recent progress in nanocarriers for direct nose to brain drug delivery. J. Drug Deliv. Sci. Technol. 64, 102642. 10.1016/j.jddst.2021.102642 - DOI
LinkOut - more resources
Full Text Sources

