Ultraviolet dosage and decontamination efficacy were widely variable across 14 UV devices after testing a dried enveloped ribonucleic acid virus surrogate for SARS-CoV-2
- PMID: 36267449
- PMCID: PMC9578676
- DOI: 10.3389/fbioe.2022.875817
Ultraviolet dosage and decontamination efficacy were widely variable across 14 UV devices after testing a dried enveloped ribonucleic acid virus surrogate for SARS-CoV-2
Abstract
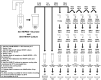
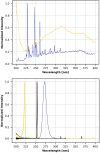
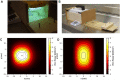
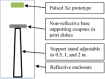
Aims: The dosages and efficacy of 14 ultraviolet (UV) decontamination technologies were measured against a SARS-CoV-2 surrogate virus that was dried onto different materials for laboratory and field testing. Methods and results: A live enveloped, ribonucleic acid (RNA) virus surrogate for SARS-CoV-2 was dried on stainless steel 304 (SS304), Navy Top Coat-painted SS304 (NTC), cardboard, polyurethane, polymethyl methacrylate (PMMA), and acrylonitrile butadiene styrene (ABS) materials at > 8.0 log10 plaque-forming units (PFU) per test coupon. The coupons were then exposed to UV radiation during both laboratory and field testing. Commercial and prototype UV-emitting devices were measured for efficacy: four handheld devices, three room/surface-disinfecting machines, five air disinfection devices, and two larger custom-made machines. UV device dosages ranged from 0.01 to 729 mJ cm-2. The antiviral efficacy among the different UV devices ranged from no decontamination up to nearly achieving sterilization. Importantly, cardboard required far greater dosage than SS304. Conclusion: Enormous variability in dosage and efficacy was measured among the different UV devices. Porous materials limit the utility of UV decontamination. Significance and impact of the study: UV devices have wide variability in dosages, efficacy, hazards, and UV output over time, indicating that each UV device needs independent technical measurement and assessment for product development prior to and during use.
Keywords: COVID-19; SARS-CoV-2; UV decontamination; decontamination; enveloped virus; enveloped virus Ф6; ultraviolet (UV).
Copyright © 2022 Buhr, Borgers-Klonkowski, Gutting, Hammer, Hamilton, Huhman, Jackson, Kennihan, Lilly, Little, Luck, Matuczinski, Miller, Sides, Yates and Young.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aps J. K. M., Martens L. C. (2005). Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci. Int. 150 (2-3), 119–131. 10.1016/j.forsciint.2004.10.026 PubMed Abstract | 10.1016/j.forsciint.2004.10.026 | Google Scholar - DOI - DOI - PubMed
-
- ASTM International (formerly the American Society for Testing and Materials) (2016). Evaluation of effectiveness of decontamination procedures for surfaces when challenged with droplets containing human pathogenic viruses. ASTM Standard Practice E2721-16. Google Scholar
-
- ASTM International (formerly the American Society for Testing and Materials) (2020). Guidance on SARS-CoV-2 surrogate selection. Available at: https://www.astm.org/COVID-19/ (Accessed on September 15, 2021). Google Scholar
-
- ASTM International (formerly the American Society for Testing and Materials) (2018b). Standard practice for evaluating efficacy of vaporous decontaminants against Bacillus spores contained within 0.2µm filter-capped tubes. ASTM Standard Practice E3092-18. Google Scholar
-
- ASTM International (formerly the American Society for Testing and Materials) (2018a). Standard practice for evaluating static and cidal chemical decontaminants against Bacillus spores using centrifugal filtration tubes. ASTM Standard Practice E3178-18. Google Scholar
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

