Functional and pharmacological role of the dopamine D4 receptor and its polymorphic variants
- PMID: 36267569
- PMCID: PMC9578002
- DOI: 10.3389/fendo.2022.1014678
Functional and pharmacological role of the dopamine D4 receptor and its polymorphic variants
Abstract
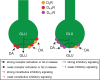
The functional and pharmacological significance of the dopamine D4 receptor (D4R) has remained the least well understood of all the dopamine receptor subtypes. Even more enigmatic has been the role of the very prevalent human DRD4 gene polymorphisms in the region that encodes the third intracellular loop of the receptor. The most common polymorphisms encode a D4R with 4 or 7 repeats of a proline-rich sequence of 16 amino acids (D4.4R and D4.7R). DRD4 polymorphisms have been associated with individual differences linked to impulse control-related neuropsychiatric disorders, with the most consistent associations established between the gene encoding D4.7R and attention-deficit hyperactivity disorder (ADHD) and substance use disorders. The function of D4R and its polymorphic variants is being revealed by addressing the role of receptor heteromerization and the relatively avidity of norepinephrine for D4R. We review the evidence conveying a significant and differential role of D4.4R and D4.7R in the dopaminergic and noradrenergic modulation of the frontal cortico-striatal pyramidal neuron, with implications for the moderation of constructs of impulsivity as personality traits. This differential role depends on their ability to confer different properties to adrenergic α2A receptor (α2AR)-D4R heteromers and dopamine D2 receptor (D2R)-D4R heteromers, preferentially localized in the perisomatic region of the frontal cortical pyramidal neuron and its striatal terminals, respectively. We also review the evidence to support the D4R as a therapeutic target for ADHD and other impulse-control disorders, as well as for restless legs syndrome.
Keywords: attention-deficit hyperactivity disorder; dopamine D4 receptor; impulsivity; polymorphic variants; restless legs syndrome.
Copyright © 2022 Ferré, Belcher, Bonaventura, Quiroz, Sánchez-Soto, Casadó-Anguera, Cai, Moreno, Boateng, Keck, Florán, Earley, Ciruela, Casadó, Rubinstein and Volkow.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, et al. . Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry (1996) 1(2):121–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

