C-type natriuretic peptide preserves central neurological function by maintaining blood-brain barrier integrity
- PMID: 36267701
- PMCID: PMC9577671
- DOI: 10.3389/fnmol.2022.991112
C-type natriuretic peptide preserves central neurological function by maintaining blood-brain barrier integrity
Abstract
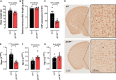
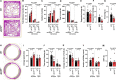
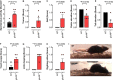
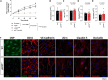
C-type natriuretic peptide (CNP) is highly expressed in the central nervous system (CNS) and key to neuronal development; however, a broader role for CNP in the CNS remains unclear. To address this deficit, we investigated behavioral, sensory and motor abnormalities and blood-brain barrier (BBB) integrity in a unique mouse model with inducible, global deletion of CNP (gbCNP-/-). gbCNP-/- mice and wild-type littermates at 12 (young adult) and 65 (aged) weeks of age were investigated for changes in gait and motor coordination (CatWalk™ and rotarod tests), anxiety-like behavior (open field and elevated zero maze tests), and motor and sensory function (modified neurological severity score [mNSS] and primary SHIRPA screen). Vascular permeability was assessed in vivo (Miles assay) with complementary in vitro studies conducted in primary murine brain endothelial cells. Young adult gbCNP-/- mice had normal gait but reduced motor coordination, increased locomotor activity in the open field and elevated zero maze, and had a higher mNSS score. Aged gbCNP-/- animals developed recurrent spontaneous seizures and had impaired gait and wide-ranging motor and sensory dysfunction. Young adult and aged gbCNP-/- mice exhibited increased BBB permeability, which was partially restored in vitro by CNP administration. Cultured brain endothelial cells from gbCNP-/- mice had an abnormal ZO-1 protein distribution. These data suggest that lack of CNP in the CNS impairs tight junction protein arrangement and increases BBB permeability, which is associated with changes in locomotor activity, motor coordination and late-onset seizures.
Keywords: C-type natriuretic peptide; ZO-1; anxiety; blood brain barrier; coordination; hyperactivity; natriuretic peptide receptor C; seizure.
Copyright © 2022 Perez-Ternero, Pallier, Tremoleda, Delogu, Fernandes, Michael-Titus and Hobbs.
Conflict of interest statement
Author AJH was a scientific advisory board member/consultant for Palatin Technologies Inc., and Novo Nordisk, and had received research support from Palatin Technologies Inc., for an unrelated project. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Barmashenko G., Buttgereit J., Herring N., Bader M., Ozcelik C., Manahan-Vaughan D., et al. (2014). Regulation of hippocampal synaptic plasticity thresholds and changes in exploratory and learning behavior in dominant negative NPR-B mutant rats. Front. Mol. Neurosci. 7:95. 10.3389/fnmol.2014.00095 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

