Reporting of Artificial Intelligence Diagnostic Accuracy Studies in Pathology Abstracts: Compliance with STARD for Abstracts Guidelines
- PMID: 36268103
- PMCID: PMC9576989
- DOI: 10.1016/j.jpi.2022.100091
Reporting of Artificial Intelligence Diagnostic Accuracy Studies in Pathology Abstracts: Compliance with STARD for Abstracts Guidelines
Abstract
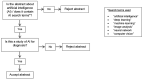
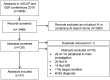
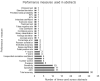
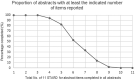
Artificial intelligence (AI) research is transforming the range tools and technologies available to pathologists, leading to potentially faster, personalized and more accurate diagnoses for patients. However, to see the use of tools for patient benefit and achieve this safely, the implementation of any algorithm must be underpinned by high quality evidence from research that is understandable, replicable, usable and inclusive of details needed for critical appraisal of potential bias. Evidence suggests that reporting guidelines can improve the completeness of reporting of research, especially with good awareness of guidelines. The quality of evidence provided by abstracts alone is profoundly important, as they influence the decision of a researcher to read a paper, attend a conference presentation or include a study in a systematic review. AI abstracts at two international pathology conferences were assessed to establish completeness of reporting against the STARD for Abstracts criteria. This reporting guideline is for abstracts of diagnostic accuracy studies and includes a checklist of 11 essential items required to accomplish satisfactory reporting of such an investigation. A total of 3488 abstracts were screened from the United States & Canadian Academy of Pathology annual meeting 2019 and the 31st European Congress of Pathology (ESP Congress). Of these, 51 AI diagnostic accuracy abstracts were identified and assessed against the STARD for Abstracts criteria for completeness of reporting. Completeness of reporting was suboptimal for the 11 essential criteria, a mean of 5.8 (SD 1.5) items were detailed per abstract. Inclusion was variable across the different checklist items, with all abstracts including study objectives and no abstracts including a registration number or registry. Greater use and awareness of the STARD for Abstracts criteria could improve completeness of reporting and further consideration is needed for areas where AI studies are vulnerable to bias.
© 2022 The Authors.
Figures
References
-
- Ioannidis J.P. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294(2):218–228. - PubMed
-
- Button K.S., Ioannidis J.P., Mokrysz C., et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. - PubMed
LinkOut - more resources
Full Text Sources

