Neoadjuvant PD-1 blockade plus chemotherapy induces a high pathological complete response rate and anti-tumor immune subsets in clinical stage III gastric cancer
- PMID: 36268179
- PMCID: PMC9578498
- DOI: 10.1080/2162402X.2022.2135819
Neoadjuvant PD-1 blockade plus chemotherapy induces a high pathological complete response rate and anti-tumor immune subsets in clinical stage III gastric cancer
Abstract
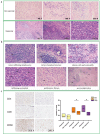
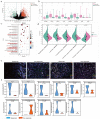
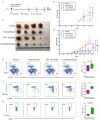
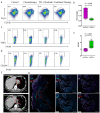
First-line PD-1 blockade plus chemotherapy significantly improves the survival benefits in late-stage gastric cancer (GC) patients. However, the pathological response rate and effects on the immune microenvironment of neoadjuvant PD-1 blockade plus chemotherapy in patients with cTNM-stage III GC remain to be elucidated. Patients with cTNM-stage III GC who underwent neoadjuvant PD-1 blockade plus chemotherapy and surgery were enrolled. Four in vivo models bearing GC were jointly established to investigate the specific roles of chemotherapy and PD-1 blockade for GC treatment. The tumor immune microenvironment was analyzed by hematoxylin and eosin (H&E) and IHC staining, multicolor flow cytometry and immunofluorescence. A total of 75 patients with cTNM-stage III (cT2-4N1-3M0) gastric cancer who received neoadjuvant PD-1 blockade plus chemotherapy (SOX/XELOX) were included in this study. After treatment, 21 (28.0%) and 57 (76.0%) patients achieved pathological complete response (pCR) and post-therapy pathological downstaging. Subgroup analyses revealed that patients with CPS >1 (32.6% vs 8.3%) and dMMR (35.7% vs 25.4%) subtype had better efficacy. Additionally, the resected specimens showed more anti-tumor immune infiltration indicating a response to neoadjuvant PD-1 blockade plus chemotherapy. Multicolor immunofluorescence and in vivo experiments on mouse models revealed that elevated M1/M2 ratio of macrophages, CD8 + T cells and plasma cells indicated effective response to treatment. Furthermore, neoadjuvant PD-1 blockade plus chemotherapy neither delayed surgery nor increased postoperative complication rate. The analyses indicate neoadjuvant PD-1 blockade plus chemotherapy is a promising therapeutic strategy in patients with cTNM-stage III GC with an encouraging pCR rate.
Keywords: cTNM-stage III gastric cancer; immune cell subpopulations; neoadjuvant PD-1 blockade plus chemotherapy; pathological complete response; tumor microenvironment.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi:10.1016/S0140-6736(18)32557-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
