Impact of Remdesivir on SARS-CoV-2 Clearance in a Real-Life Setting: A Matched-Cohort Study
- PMID: 36268521
- PMCID: PMC9578770
- DOI: 10.2147/DDDT.S369473
Impact of Remdesivir on SARS-CoV-2 Clearance in a Real-Life Setting: A Matched-Cohort Study
Abstract
Background: Evidence regarding the impact of remdesivir (RDV) on SARS-CoV-2 viral clearance (VC) is scarce. The aim of this study was to compare VC timing in hospitalized COVID-19 patients who did or did not receive RDV.
Methods: This was a matched-cohort study of patients hospitalized with pneumonia, a SARS-CoV-2-positive nasopharyngeal swab (NPS) at admission, and at least one NPS during follow-up. Patients who received RDV (cases) and those who did not (controls) were matched in a 1:2 ratio by age, sex, and PaO2/FiO2 (P/F) values at admission. NPSs were analyzed using real-time polymerase chain reaction. Time to VC (within 30 days after hospital discharge) was estimated using the Kaplan-Meier curve. A multivariable Cox proportional hazard model was fitted to determine factors associated with VC.
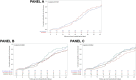
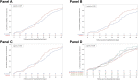
Results: There were 648 patients enrolled in the study (216 cases and 432 controls). VC was observed in 490 patients (75.6%), with a median time of 25 (IQR 16-34) days. Overall, time to VC was similar between cases and controls (p = 0.519). However, time to VC was different when considering both RDV treatment status and age (p = 0.007). A significant finding was also observed when considering both RDV treatment status and P/F values at admission (p = 0.007). A multivariate analysis showed that VC was associated with a younger age (aHR = 0.990, 95% CI 0.983-0.998 per every 10-year increase in age; p = 0.009) and a higher baseline P/F ratio (aHR=1.275, 95% CI 1.029-1.579; p=0.026), but not with RDV treatment status.
Conclusion: Time to VC was similar in cases and controls. However, there was a benefit associated with using RDV in regard to time to VC in younger patients and in those with a P/F ratio ≤200 mmHg at hospital admission.
Keywords: COVID-19; SARS-CoV-2; remdesivir; viral clearance.
© 2022 Spagnuolo et al.
Conflict of interest statement
Dr Vincenzo Spagnuolo reports grants from Gilead Sciences Srl, during the conduct of the study. Dr Chiara Oltolini reports grants from Gilead Sciences, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures
References
-
- COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed June 29, 2022. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

