A review of microbial-environmental interactions recorded in Proterozoic carbonate-hosted chert
- PMID: 36268586
- PMCID: PMC10092529
- DOI: 10.1111/gbi.12527
A review of microbial-environmental interactions recorded in Proterozoic carbonate-hosted chert
Abstract
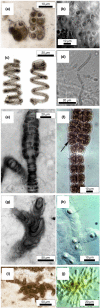
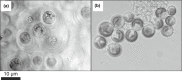
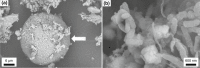
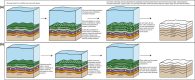
The record of life during the Proterozoic is preserved by several different lithologies, but two in particular are linked both spatially and temporally: chert and carbonate. These lithologies capture a snapshot of dominantly peritidal environments during the Proterozoic. Early diagenetic chert preserves some of the most exceptional Proterozoic biosignatures in the form of microbial body fossils and mat textures. This fossiliferous and kerogenous chert formed in shallow marine environments, where chert nodules, layers, and lenses are often surrounded by and encased within carbonate deposits that themselves often contain kerogen and evidence of former microbial mats. Here, we review the record of biosignatures preserved in peritidal Proterozoic chert and chert-hosting carbonate and discuss this record in the context of experimental and environmental studies that have begun to shed light on the roles that microbes and organic compounds may have played in the formation of these deposits. Insights gained from these studies suggest temporal trends in microbial-environmental interactions and place new constraints on past environmental conditions, such as the concentration of silica in Proterozoic seawater, interactions among organic compounds and cations in seawater, and the influence of microbial physiology and biochemistry on selective preservation by silicification.
Keywords: biosignature; carbonate; chert; fossil; proterozoic.
© 2022 The Authors. Geobiology published by John Wiley & Sons Ltd.
Figures







References
-
- Aitken, J. D. (1988). Giant “algal” reefs, Middle/Upper Proterozoic Little Dal Group (>770, >1200 Ma), Mackenzie Mountains, N.W.T., Canada. Reef: Canada and Adjacent Areas Memoir, 13, 13–23.
-
- Aitken, J. D. , & Narbonne, G. M. (1989). Two occurrences of Precambrian thrombolites from the Mackenzie Mountains, Northwestern Canada. PALAIOS, 4, 384–388. 10.2307/3514563 - DOI
-
- Alleon, J. , Bernard, S. , Le Guillou, C. , Marin‐Carbonne, J. , Pont, S. , Beyssac, O. , McKeegan, K. D. , & Robert, F. (2016). Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and mineralogy. Nature Communications, 7, 11977. 10.1038/ncomms11977 - DOI - PMC - PubMed
-
- Amard, B. , & Bertrand‐Sarfati, J. (1997). Microfossils in 2000 Ma old cherty stromatolites of the Franceville Group, Gabon. Precambrian Research, 81, 197–221.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

