Topical corticosteroids for dry eye
- PMID: 36269562
- PMCID: PMC9586197
- DOI: 10.1002/14651858.CD015070.pub2
Topical corticosteroids for dry eye
Abstract
Background: Dry eye disease (DED), arising from various etiologic factors, leads to tear film instability, ocular surface damage, and neurosensory changes. DED causes symptoms such as ocular dryness, burning, itching, pain, and visual impairment. Given their well-established anti-inflammatory effects, topical steroid preparations have been widely used as a short-term treatment option for DED. Because of potential risks of ocular hypertension, cataracts, and infections associated with the long-term use of topical steroids, published trials comparing the efficacy and safety of topical steroids (versus placebo) have mostly been of short duration (three to eight weeks).
Objectives: To evaluate the effectiveness and safety of topical corticosteroids compared with no treatment, placebo, other steroidal or non-steroidal therapies, or a combination of therapies for DED.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL, which contains the Cochrane Eyes and Vision Trials Register; 2021, Issue 8); Ovid MEDLINE; Ovid Embase; Latin American and Caribbean Health Sciences database (LILACS); ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), without restriction on language or year of publication. The date of the last search was 20 August 2021.
Selection criteria: We included randomized controlled trials (RCTs) in which topical corticosteroids, alone or in combination with tobramycin, were compared with no treatment, artificial tears (AT), vehicles, AT plus tobramycin, or cyclosporine A (CsA).
Data collection and analysis: We applied standard Cochrane methodology.
Main results: We identified 22 RCTs conducted in the USA, Italy, Spain, China, South Korea, and India. These RCTs reported outcome data from a total of 4169 participants with DED. Study characteristics and risk of bias All trials recruited adults aged 18 years or older, except one trial that enrolled children and adolescents aged between 3 and 14 years. Half of these trials involved predominantly female participants (median 79%, interquartile range [IQR] 76% to 80%). On average, each trial enrolled 86 participants (IQR 40 to 158). The treatment duration of topical steroids ranged between one week and three months; trial duration lasted between one week and six months. Eight trials were sponsored exclusively by industry, and four trials were co-sponsored by industry and institutional or governmental funds. We assessed the risk of bias of both subjective and objective outcomes using RoB 2, finding nearly half of the trials to be at high risk of bias associated with selective outcome reporting. Findings Of the 22 trials, 16 evaluated effects of topical steroids, alone or in combination with tobramycin, as compared with lubricants (AT, vehicle), AT plus tobramycin, or no treatment. Corticosteroids probably have a small to moderate effect on improving patient-reported symptoms by 0.29 standardized mean difference (SMD) (95% confidence interval [CI] 0.16 to 0.42) as compared with lubricants (moderate certainty evidence). Topical steroids also likely have a small to moderate effect on lowering corneal staining scores by 0.4 SMDs (95% CI 0.18 to 0.62) (moderate certainty evidence). However, steroids may increase tear film break-up time (TBUT) slightly (mean difference [MD] 0.70 s, 95% CI 0.06 to 1.34; low certainty evidence) but not tear osmolarity (MD 1.60 mOsm/kg, 95% CI -10.47 to 13.67; very low certainty evidence). Six trials examined topical steroids, either alone or in combination with CsA, against CsA alone. Low certainty evidence indicates that steroid-based interventions may have a small to moderate effect on improving participants' symptoms (SMD -0.33, 95% CI -0.51 to -0.15), but little to no effect on corneal staining scores (SMD 0.05, 95% CI -0.25 to 0.35) as compared with CsA. The effect of topical steroids compared to CsA alone on TBUT (MD 0.37 s, 95% CI -0.13 to 0.87) or tear osmolarity (MD 5.80 mOsm/kg, 95% CI -0.94 to 12.54; loteprednol etabonate alone) is uncertain because the certainty of the evidence is low or very low. None of the included trials reported on quality of life scores. Adverse effects The evidence for adverse ocular effects of topical corticosteroids is very uncertain. Topical corticosteroids may increase participants' risk of intraocular pressure (IOP) elevation (risk ratio [RR] 5.96, 95% CI 1.30 to 27.38) as compared with lubricants. However, when compared with CsA, steroids alone or combined with CsA may decrease or increase IOP elevation (RR 1.45, 95% CI 0.25 to 8.33). It is also uncertain whether topical steroids may increase risk of cataract formation when compared with lubricants (RR 0.34, 95% CI 0.01 to 8.22), given the short-term use and study duration (four weeks or less) to observe longer-term adverse effects. AUTHORS' CONCLUSIONS: Overall, the evidence for the specified review outcomes was of moderate to very low certainty, mostly due to high risk of bias associated with selective results reporting. For dry eye patients whose symptoms require anti-inflammatory control, topical corticosteroids probably provide small to moderate degrees of symptom relief beyond lubricants, and may provide small to moderate degrees of symptom relief beyond CsA. However, the current evidence is less certain about the effects of steroids on improved tear film quality or quantity. The available evidence is also very uncertain regarding the adverse effects of topical corticosteroids on IOP elevation or cataract formation or progression. Future trials should generate high certainty evidence to inform physicians and patients of the optimal treatment strategies with topical corticosteroids in terms of regimen (types, formulations, dosages), duration, and its time-dependent adverse profile.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Su‐Hsun Liu: reports a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution.Ian J Saldanha: reports grants and contracts, Cochrane Eyes and Vision, from National Eye Institute, National Institutes of Health, USA; payment to institution. Travel reimbursement for making a talk on outcomes related to dry eye in 2018 from Johns Hopkins Wilmer Eye Institute; personal payment.Alison G Abraham: declared that she has no conflicts of interest.Thanitsara Rittiphairoj: declared that she has no conflicts of interest.Scott Hauswirth: reports grants and contracts for paid investigator from TearSolutions and Sylentis (contract pending—study not yet underway as of 23 February 2021); paid to institution. Payments for presentations from Kala Pharmaceuticals, Dompe, Sun Pharmaceuticals, Takeda, and Avedro; personal payment. Support for INTREPID meeting (indirect) from Alcon/Novartis; personal payment. Stock shares in Oyster Point (paid for and owned individually, not as compensation), TearRestore (compensation for design and medical advisory work), and Horizon Pharma (paid for and owned individually, not as compensation); personal payment. Consulting fees for study design and analysis from Ocular Therapeutix (pending); personal payment. Advisory Board payments from Dompe, NuSight Medical, Kala Pharmaceuticals, Sun Pharmaceuticals, EyePoint Medical, EyeVance Pharma, Horizon Pharma, Avedro/Glaukos, Quidel, and Sight Sciences; personal payment. Writing assistance from Takeda (medical writer for review paper); personal payment. SH reports publishing opinions on topical immunomodulation in dry eye in the OSDocs Facebook group (moderator/co‐administrator role), and published 'When dry eye compromises corneal integrity' inReview of Optometry , Nov 2017 (contract pending—study not yet underway as of 23 February 2021). They report working as an Optometrist at the University of Colorado.Darren Gregory: reports working as an Ophthalmologist at the University of Colorado Eye Center. Their clinical work focuses on the treatment of dry eyes, which sometimes involves the use of topical corticosteroid medications.Cristos Ifantides: reports being an inventor with intellectual property assigned to their university. The design relates to dry eye, but does not relate to corticosteroid use for dry eye. It is a design for a new form of eyeglasses that can theoretically help with dry eye (patent application filed, University of Colorado), paid to institution. Ownership of stock in Pfizer. Their partner works for AbbVie, which makes medications related to dry eye. She does not work in the eye care space. CI reports working as a clinician at Denver Health and University of Colorado, where they are an attending physician.Tianjing Li: reports a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution.
Figures
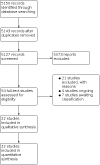





















































Update of
- doi: 10.1002/14651858.CD015070
References
References to studies included in this review
Akhlaq 2019 {published data only}
-
- Akhlaq A, Kheirkhah A, Aggarwal S, Cavalcanti B, Mueller R, Abbouda A, et al. Patients enrichment for increased dendritiform cells using in vivo confocal microscopy results in improved response to topical steroids in dry eye disease: results of the therapeutic response to antiinflammatory agents in the corneal epithelium (TRACE) study. Investigative Ophthalmology & Visual Science 2019;60:6753.
-
- Hamrah P, Akhlaq A, Ozmen MC, Kheirkhah A, Aggarwal S, Cavalcanti B, et al. Change in dendritiform cell density by in vivo confocal microscopy may be used as a surrogate biomarker for therapeutic response in ery eye disease patients enriched for presence of inflammation: results from the therapeutic response to anti-inflammatory. Investigative Ophthalmology & Visual Science 2019;60:5207.
-
- NCT02106377. Using in vivo confocal microscopy to assess cellular response and efficacy of steroid treatment in ery eye disease. clinicaltrials.gov/ct2/show/NCT02106377 (first received 18 April 2014).
-
- NCT02120079. The utility of IVCM to assess cellular response and efficacy of long-term topical steroid treatment in patients With DED. clinicaltrials.gov/show/NCT02120079 (first received 22 April 2014).
Aragona 2013 {published data only}
-
- Aragona P, Spinella R, Rania L, Postorino E, Sommario MS, Roszkowska AM, et al. Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjögren syndrome. European Journal of Ophthalmology 2013;23(3):368‐76. - PubMed
Avunduk 2003 {published data only}
-
- Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. American Journal of Ophthalmology 2003;136(4):593‐602. - PubMed
-
- Chan CK, Lam DS. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. American Journal of Ophthalmology 2004;137(6):1157-8. - PubMed
Bausch 2013 {published data only}
-
- NCT01817582. Lotemax® Gel 0.5% and Restasis 0.05% in participants with mild or moderate keratoconjunctivitis sicca (ery eye disease). clinicaltrials.gov/show/NCT01817582 (first received 25 March 2013).
Byun 2012 {published data only}
-
- Byoun Y, Kim S, Kim TI, Kim E. Efficacy of combined treatment of cyclosporine 0.05% and 1% methylprednisolone on dry eye patients of Sjögren syndrome. Investigative Ophthalmology & Visual Science 2007;48:ARVO E‐Abstract 401.
-
- Byun YJ, Kim TI, Kwon SM, Seo KY, Kim SW, Kim EK, et al. Efficacy of combined 0.05% cyclosporine and 1% methylprednisolone treatment for chronic dry eye. Cornea 2012;31(5):509-13. - PubMed
Cao 2018 {published data only}
-
- Cao XY, He L, Li Y H, Li Y. Study on the effect of sodium hyaluronate combined with loteprednol eye drops on the treatment of dry eye in children. International Eye Science 2018;18(3):516‐9.
Chen 2020 {published data only}
-
- Chen W, Shi XL, He XH, Mao YH, Li C, Dong N. Loteprednol combined with sodium hyaluronate in the treatment of dry eye disease and its effect on tnf-α and cxcl10 in tears. Journal of Biological Regulators and Homeostatic Agents 2020;34(5):1825‐9. - PubMed
KPI‐121 (Phase 2) {published data only}
-
- NCT02188160. Safety and efficacy of KPI-121 in subjects with dry eye disease (Kauai). clinicaltrials.gov/show/NCT02188160 (first received 11 July 2014).
KPI‐121 (STRIDE1) {published data only}
-
- NCT02813265. Safety and efficacy of KPI-121 in subjects with dry eye disease. clinicaltrials.gov/show/NCT02813265 (first received 24 June 2016).
KPI‐121 (STRIDE2) {published data only}
-
- NCT02819284. Safety and efficacy of KPI-121 compared to placebo in subjects with dry eye disease. clinicaltrials.gov/show/NCT02819284 (first received 30 June 2016).
KPI‐121 (STRIDE3) {published data only}
-
- NCT03616899. Safety and efficacy of KPI-121 in subjects with DED. clinicaltrials.gov/show/NCT03616899 (first received 6 August 2018).
Lee 2014 {published data only}
-
- Lee H, Chung B, Kim KS, Seo KY, Choi BJ, Kim TI. Effects of topical loteprednol etabonate on tear cytokines and clinical outcomes in moderate and severe meibomian gland dysfunction: randomized clinical trial. American Journal of Ophthalmology 2014;158(6):1172‐83. e1. - PubMed
-
- NCT01692652. Changes of inflammatory cytokines in the tears of moderate and severe MGD treated with topical loteprednol etabonate. clinicaltrials.gov/show/NCT01692652 (first received 25 September 2012).
Li 2021 {published data only}
-
- Li N, Lai J. Effects of fluorometholone combined with sodium hyaluronate eye drops in the treatment of xerophthalmia and the influence on inflammatory factors in tears. International Eye Science 2021;21(3):509‐14.
Lin 2015 {published data only}
Luo 2013 {published data only}
-
- Luo XL, Lei C, Wang BL. Clinical study of corticosteriod for meibomian gland dysfunction. International Eye Science 2013;13(2):377-9.
NCT01276223 {published data only}
-
- NCT01276223. Evaluation of anti-inflammatory treatment in dry eye patients. clinicaltrials.gov/ct2/show/NCT01276223 (first received 12 January 2011).
Pflugfelder 2004 {published data only}
-
- Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, De Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. American Journal of Ophthalmology 2004;138(3):444‐57. - PubMed
Pinto‐Fraga 2016 {published data only}
-
- Aapola U, Nattinen J, Jylha A, Pinto-Fraga J, Lpez-Miguel A, Gonzalez-Garcia MJ, et al. Tear fluid proteome reveals inflammation and immune response proteins as potential predictive biomarkers of the effects of desiccating stress and dry eye treatments. Investigative Ophthalmology & Visual Science 2016;57(12):397.
-
- Calonge M, Pinto-Fraga J, Enriquez-De-Salamanca A, Fernandez I, Gonzalez-Garcia MJ, Lopez-Miguel A, et al. Tear cytokine biomarkers in dry eye patients subjected to environmental stress and treated with topical 0.1% fluorometholone. Investigative Ophthalmology & Visual Science 2016;57(12):2861.
-
- Estevez J, Pesudovs K. Re: Pinto-Fraga et al.: topical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: a randomized controlled clinical trial. Ophthalmology 2017;124(2):e14. - PubMed
-
- Nättinen J, Jylhä A, Aapola U, Enríquez-de-Salamanca A, Pinto-Fraga J, López-Miguel A, et al. Topical fluorometholone treatment and desiccating stress change inflammatory protein expression in tears. Ocular Surface 2018;16(1):84‐92. - PubMed
-
- NCT02051023. Efficacy and safety of fluorometholone (FML) in dry eye disease (keratoconjunctivitis sicca). clinicaltrials.gov/show/NCT02051023 (first received 31 January 2014).
Qazi 2015 {published data only}
-
- Efficacy of Zylet vs. Lotemax for the treatment of ocular surface inflammation/MGD/blepharitis. clinicaltrials.gov/show/NCT01456780 (first received 21 October 2011).
-
- Qazi Y, Kheirkhah A, Dohlman TH, Cruzat A, Cavalcanti B, Colon C, et al. Corneal dendritic cells as a surrogate biomarker of therapeutic efficacy in dry eye-associated corneal inflammation. Investigative Ophthalmology & Visual Science 2015;56(7):291.
Sheppard 2014 {published data only}
-
- Donnenfeld ED, Sheppard JD, Holland EJ, Slonim CH, Solomon R, Solomon KD, et al. Prospective, multicenter, randomized controlled study on the effect of loteprednol etabonate on initiating therapy with cyclosporin A. In: Annual Meeting of American Academy of Ophthalmology; 2007 Nov 10-13; New Orleans (LA). 2007.
-
- NCT00407043. Multicenter, randomized, controlled study of the effect of Lotemax on initiation of dry eye treatment with Restasis. clinicaltrials.gov/show/NCT00407043 (first received 4 December 2006).
-
- Sheppard JD, Donnenfeld ED, Holland EJ, Slonim CB, Solomon R, Solomon KD, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye & Contact Lens 2014;40(5):289‐96. - PubMed
-
- Sheppard JD, Donnenfeld ED. Topical loteprednol 0.5% induction therapy improves topical cyclosporine emulsion tolerability in chronic dry eye disease. Investigative Ophthalmology & Vision Science 2008;49:ARVO E‐Abstract 99.
Singla 2019 {published data only}
Wan 2012 {published data only}
-
- Wan PX, Wang XR, Song YY, Li ZY, Duan HC, Zhang W, et al. Study on the treatment of dry eye with loteprednol etabonate. Zhonghua Yan Ke Za Zhi [Chinese Journal of Ophthalmology] 2012;48(2):142‐7. - PubMed
References to studies excluded from this review
Abud 2016 {published data only}
Acord 2010 {published data only}
-
- Acord C, Gonzales A, McKee AG. Loteprednol etabonate 0.2% in the possible treatment of dry eye syndrome. Optometry 2010;81:299‐300.
Asbell 2011 {published data only}
Boynton 2015 {published data only}
-
- Boynton GE, Raoof D, Niziol LM, Hussain M, Mian SI. Prospective randomized trial comparing efficacy of topical loteprednol etabonate 0.5% versus cyclosporine-a 0.05% for treatment of dry eye syndrome following hematopoietic stem cell transplantation. Cornea 2015;34(7):725‐32. - PubMed
ChiCTR‐IPQ‐15006773 {published data only}
-
- ChiCTR-IPQ-15006773. 0.1% tacrolimus (FK506) in the treatment of moderately severe dry eye clinical efficacy evaluation. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IPQ-15006773 (first received 27 March 2015).
Edward 2014 {published data only}
-
- NCT02028312. A phase IV, randomized, parallel group, investigator-masked evaluation of the effect of loteprednol etabonate ophthalmic gel 0.5% on the initiation of dry eye treatment with Restasis®. clinicaltrials.gov/show/NCT02028312 (first received 7 January 2014).
EUCTR2006‐003391‐35‐NL {published data only}
-
- EUCTR2006-003391-35-NL. Evaluation of the efficacy and safety of unpreserved dexamethasone phosphate 0.1% eye drops (T1910) versus placebo in patients with bilateral treated severe keratoconjunctivitis sicca due to Sjögrens' syndrome. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-003391-35-NL (first received 7 May 2006).
EUCTR2019‐000747‐27‐IT {published data only}
-
- EUCTR2019-000747-27-IT. A clinical trial to evaluate the safety and efficacy of Pro-ocular™ topical gel in two different concentration, 0.5% and 1%, when administered in the forehead twice a day for 12 weeks in patients diagnosed with dry eye syndrome. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-000747-27-IT (first received 10 July 2020).
Gupta 2021 {published data only}
ISRCTN13765551 {published data only}
-
- ISRCTN13765551. Comparison of the treatment effect of preservative-free vs preserved eye drops in patients with dry eye syndrome. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN13765551 (first received 2 May 2014).
Jee 2014 {published data only}
-
- Jee D, Park SH, Kim MS, Kim EC. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome treated with preservative-free versus preserved eye drops. Investigative Ophthalmology & Vision Science 2014;55(8):5081‐9. - PubMed
JPRN‐UMIN000025159 {published data only}
-
- JPRN-UMIN000025159. Efficacy of expression treatment on o-MGD (obstructive meibomian gland dysfunction). trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000025159 (first received 2 January 2017).
Kallab 2020 {published data only}
-
- Kallab M, Szegedi S, Hommer N, Stegmann H, Kaya S, Werkmeister RM, et al. Topical low dose preservative-free hydrocortisone reduces signs and symptoms in patients with chronic dry eye: a randomized clinical trial. Advances in Therapy 2020;37(1):329‐41. - PubMed
Korenfeld 2021 {published data only}
-
- Korenfeld M, Nichols KK, Goldberg D, Evans D, Sall K, Foulks G, et al. Safety of KPI-121 ophthalmic suspension 0.25% in patients with dry eye disease: a pooled analysis of 4 multicenter, randomized, vehicle-controlled studies. Cornea 2021;40(5):564-70. - PubMed
Lee 2006 {published data only}
-
- Lee HK, Ryu IH, Seo KY, Hong S, Kim HC, Kim EK. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology 2006;113(2):198‐205. - PubMed
NCT03907865 {published data only}
-
- NCT03907865. Clinical efficacy of topical hydrocortisone 0.335% (Softacort®) in patients with chronic dry eye disease and associated ocular surface inflammation. clinicaltrials.gov/show/NCT03907865 (first received 9 April 2019).
Rolando 2008 {published data only}
-
- Rolando M, Solignani F, Valente C, Allavena F, Bertolotto M, Barabino S. Is there a role for a long term tapered small dose steroidal treatment for keratoconjunctivitis sicca? Investigative Ophthalmology & Vision Science 2008;49:ARVO E‐Abstract 97.
Ryu 2005 {published data only}
-
- Ryu I, Lee HK, Seo KR, Kim E. Change of nerve growth factor after 01% prednisolone instillation in dry eye syndrome patients and its correlation with clinical parameters. Investigative Ophthalmology & Vision Science 2005;46:ARVO E‐Abstract 2049.
Shen 2015 {published data only}
-
- Shen ZB, Li JL. Clinical effect of 1g/L fluorometholone drops combined with soft corneal contact lens for filamentary keratitis. International Eye Science 2015;15(9):1633‐5.
Sindhu 2015 {published data only}
-
- Sindhu S, Dutta S, Beg MA, Mittal SK, Gupta SD. Comparative evaluation of topical carboxymethyl cellulose either alone or in combination with topical corticosteroid in the treatment of dry eye in a tertiary-care teaching hospital. National Journal of Physiology, Pharmacy and Pharmacology 2015;5(3):207-11.
Wong 2021 {published data only}
-
- Wong S (Editor). Loteprednol 0.25% (Eysuvis) for dry eye disease. Medical Letter on Drugs and Therapeutics 2021;63(1624):75-7. - PubMed
References to studies awaiting assessment
ChiCTR‐IPR‐15007196 {published data only (unpublished sought but not used)}
-
- ChiCTR-IPR-15007196. Clinical trial of sodium bromide hydrate eye drops and Pranoprofen eye drops for dry eye. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IPR-15007196 (first received 9 October 2015).
Herman 2005 {published data only (unpublished sought but not used)}
-
- Herman JP, Korb DR, Greiner JV, Scaffidi RC, Blackie CA. Treatment of lid wiper epitheliopathy with a metastable lipid emulsion or a corticosteroid. Investigative Ophthalmology & Vision Science 2005;46:ARVO E‐Abstract 2017.
NCT00471419 {published data only (unpublished sought but not used)}
-
- NCT00471419. Phase II study of AL-2178 (FID 109980) in the treatment of dry eye. clinicaltrials.gov/show/NCT00471419 (first received 7 May 2007).
NCT00560638 {published data only (unpublished sought but not used)}
-
- NCT00560638. Loteprednol etabonate ophthalmic suspension for the treatment of dry eye. clinicaltrials.gov/ct2/show/NCT00560638 (first received 19 November 2007).
NCT01562795 {published data only (unpublished sought but not used)}
-
- NCT01562795. Efficacy of nonsteroidal anti-inflammatory drugs in treatment of moderate and severe dry eye disease. clinicaltrials.gov/ct2/show/NCT01562795 (first received 23 March 2012).
NCT03418727 {published data only (unpublished sought but not used)}
-
- NCT03418727. Dry eye disease study with brimonidine. clinicaltrials.gov/show/NCT03418727 (first received 1 February 2018).
NTR2291 {published data only (unpublished sought but not used)}
-
- NTR2291. Ocular inflammation and dry eye. trialsearch.who.int/?trialid=NTR2291 (first received 15 April 2010).
References to ongoing studies
CTRI/2021/02/031182 {published data only (unpublished sought but not used)}
-
- CTRI/2021/02/031182. Eye drops made of antibodies for dry eye disease patients. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/02/031182 (first received 10 February 2021).
ISRCTN16288419 {published data only (unpublished sought but not used)}
-
- ISRCTN16288419. Evaluation of the performance of new substitute tears in dry eye patients. trialsearch.who.int/?TrialID=ISRCTN16288419 (first received 8 May 2020).
NCT04734197 {published data only}
-
- NCT04734197. A Research Study To See How Well an Eye Drop, SURF-100 (A Mycophenolic Acid/Betamethasone Sodium Phosphate Combination), Works and What Side Effects There Are in Subjects With Dry Eye Disease. clinicaltrials.gov/show/NCT04734197 (first received 2 February 2021).
NCT04734210 {published data only}
-
- NCT04734210. A research study to see how well an eye drop, SURF-200 (0.02% and 0.04% Betamethasone Sodium Phosphate), works, what side effects there are, and to compare it with vehicle (placebo) in subjects diagnosed with dry eye disease and experiencing an episodic flare-up. clinicaltrials.gov/show/NCT04734210 (first received 2 February 2021).
Additional references
Altman 1996
Avunduk 2003
-
- Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. American Journal of Ophthalmology 2003;136(4):593-602. - PubMed
Barabino 2011
-
- Barabino S, Montaldo E, Corsi E, Valente C, Solignani F, Mingari MC, et al. The effect of tapered small dose steroidal treatment on symptoms, clinical signs, and ocular surface inflammation in patients with dry eye syndrome. Investigative Ophthalmology & Vision Science 2011;52:3826.
Beckman 2020
Bhaskaran 2014
Bron 2003
-
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22:640-50. - PubMed
Bron 2017
-
- Bron AJ, Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocular Surface 2017;15(3):438-510. - PubMed
Byun 2012
-
- Byun YJ, Kim TI, Kwon SM, Seo KY, Kim SW, Kim EK, et al. Efficacy of combined 0.05% cyclosporine and 1% methylprednisolone treatment for chronic dry eye. Cornea 2012;31(5):509-13. - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Lawrence Erlbaum Associates Inc, 1988.
Comstock 2018
-
- Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opinion on Pharmacotherapy 2018;19(4):337-53. - PubMed
Covidence [Computer program]
-
- Covidence. Version accessed 9 August 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Cutolo 2019
-
- Cutolo CA, Barabino S, Bonzano C, Traverso CE. The use of topical corticosteroids for treatment of dry eye syndrome. Ocular Immunology and Inflammation 2019;27(2):266-75. - PubMed
Deeks 2021
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
De Paiva 2006a
-
- De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Experimental Eye Research 2006;83(3):526-35. [DOI: 10.1016/j.exer.2006.02.004] - DOI - PubMed
De Paiva 2006b
-
- De Paiva SC, Corrales RM, Villarreal AL, Farley W, Li DQ, Stern ME, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Investigative Ophthalmology and Vision Science 2006;47(7):2847-56. [DOI: 10.1167/iovs.05-1281] - DOI - PubMed
De Paiva 2019
DEQS
Downie 2017
Ervin 2017
Evans 2017
-
- Evans DG, Sheppard JD, Williams JI. Loteprednol etabonate ophthalmic gel 0.5% for inflammation associated with dry eye disease: outcomes of a 12-week Phase 2 clinical study. In: Annual Meeting of the American Optometric Association; 2017 Sep 27-30; Washington, DC. 2017.
Gomes 2017
-
- Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, et al. TFOS DEWS II iatrogenic report. Ocular Surface 2017;15(3):511-38. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. - PubMed
Higgins 2021a
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
-
- Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Holland 2019
IReST
Jones 2002
-
- Jones L, MacDougall N, Sorbara LG. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optomery and Visual Science 2002;79:753-61. - PubMed
Jones 2017
-
- Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy Report. Ocular Surface 2017;15(3):575-628. - PubMed
Kojima 2020
Lee 2006
Legge 1989
-
- Legge GE, Ross JA, Luebker A, Lamay J. Psychophysics of reading: VIII. The Minnesota low-vision reading test. Optometry and Vision Science 1989;66:843-53. - PubMed
Lekhanont 2007
Lemp 1995
-
- Lemp MA. Report of the National Eye Institute/Industry Workshop on clinical trials in dry eyes. CLAO Journal 1995;21:211-32. - PubMed
Li 2021
-
- Li T, Higgins JPT, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Macri 2000
-
- Macri A, Rolando M, Pflugfelder S. A standardized visual scale for evaluation of tear fluorescein clearance. Ophthalmology 2000;107:1338-43. - PubMed
Messmer 2015
Miller 2010
-
- Miller KL, Walt JG, Mink DR, Satram-Hoang S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. Archives of Ophthalmology 2010;128(1):94-101. - PubMed
Nelson 2017
Newman‐Casey 2018
Ngo 2013
-
- Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013;32(9):1204-10. - PubMed
Nichols 2016
-
- Nichols KK, Bacharach J, Holland E, Kislan T, Shettle L, Lunascek O, et al. Impact of dry eye disease on work productivity, and patients' satisfaction with over-the-counter dry eye treatments. Investigative Ophthalmology and Vision Science 2016;57(7):2975-82. [DOI: 10.1167/iovs.16-19419] - DOI - PubMed
Oden 1998
-
- Oden NL, Lilienfeld DE, Lemp MA, Nelson JD, Ederer F. Sensitivity and specificity of a screening questionnaire for dry eye. Advances in Experimental Medicine Biology 1998;438:807-20. - PubMed
Page 2021
-
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Pan 2017
Pflugfelder 2004
-
- Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. American Journal of Ophthalmology 2004;138(3):444-57. [DOI: 10.1016/j.ajo.2004.04.052] - DOI - PubMed
Pucker 2016
Qiu 2011
-
- Qiu X, Gong L, Sun X, Jin H. Age-related variations of human tear meniscus and diagnosis of dry eye with Fourier-domain anterior segment optical coherence tomography. Cornea 2011;30:543-9. - PubMed
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.10.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Saldanha 2017
Saldanha 2018
Schiffman 2000
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2019
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Shanti 2020
Sheppard 2014
-
- Sheppard JD, Torkildsen GL, Lonsdale JD, D'Ambrosio FA Jr, McLaurin EB, Eiferman RA, et al, OPUS-1 Study Group. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 Phase 3 Study. Clinical Trial 2014;121(2):475-83. [DOI: 10.1016/j.ophtha.2013.09.015] - DOI - PubMed
Shih 2017
Stapleton 2017
-
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocular Surface 2017;15(3):334-65. - PubMed
Stephenson 2016
-
- Stephenson L, Mistry V, Spink G, Morrison D, Thomas A, Barton S, et al. The management of dry eye. Drug and Therapeutics Bulletin 2016;54(1):9-12. - PubMed
WebPlotDigitizer [Computer program]
-
- WebPlotDigitizer. Version 4.5. Rohatgi A, accessed 22 February 2022. Available from: automeris.io/WebPlotDigitizer.
Wolffsohn 2017
-
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocular Surface 2017;15:539-74. - PubMed
Yu 2011
-
- Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 2011;30(4):379-87. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

