Restrictive cardiomyopathy: definition and diagnosis
- PMID: 36269634
- PMCID: PMC9712030
- DOI: 10.1093/eurheartj/ehac543
Restrictive cardiomyopathy: definition and diagnosis
Abstract
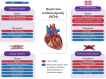
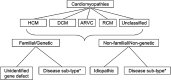
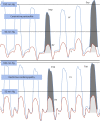
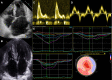
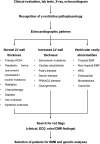
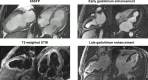
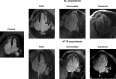
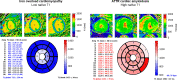
Restrictive cardiomyopathy (RCM) is a heterogeneous group of diseases characterized by restrictive left ventricular pathophysiology, i.e. a rapid rise in ventricular pressure with only small increases in filling volume due to increased myocardial stiffness. More precisely, the defining feature of RCM is the coexistence of persistent restrictive pathophysiology, diastolic dysfunction, non-dilated ventricles, and atrial dilatation, regardless of ventricular wall thickness and systolic function. Beyond this shared haemodynamic hallmark, the phenotypic spectrum of RCM is wide. The disorders manifesting as RCM may be classified according to four main disease mechanisms: (i) interstitial fibrosis and intrinsic myocardial dysfunction, (ii) infiltration of extracellular spaces, (iii) accumulation of storage material within cardiomyocytes, or (iv) endomyocardial fibrosis. Many disorders do not show restrictive pathophysiology throughout their natural history, but only at an initial stage (with an evolution towards a hypokinetic and dilated phenotype) or at a terminal stage (often progressing from a hypertrophic phenotype). Furthermore, elements of both hypertrophic and restrictive phenotypes may coexist in some patients, making the classification challenge. Restrictive pathophysiology can be demonstrated by cardiac catheterization or Doppler echocardiography. The specific conditions may usually be diagnosed based on clinical data, 12-lead electrocardiogram, echocardiography, nuclear medicine, or cardiovascular magnetic resonance, but further investigations may be needed, up to endomyocardial biopsy and genetic evaluation. The spectrum of therapies is also wide and heterogeneous, but disease-modifying treatments are available only for cardiac amyloidosis and, partially, for iron overload cardiomyopathy.
Keywords: Amyloidosis; Classification; Myocardial disease; RCM; Restrictive cardiomyopathy.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared.
Figures


References
-
- Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270–276. - PubMed
-
- Knight DS, Zumbo G, Barcella W, Steeden JA, Muthurangu V, Martinez-Naharro A, et al. Cardiac structural and functional consequences of amyloid deposition by cardiac magnetic resonance and echocardiography and their prognostic roles. JACC Cardiovasc Imaging 2019;12:823–833. - PubMed
-
- Goldstein JA, Kern MJ. Hemodynamics of constrictive pericarditis and restrictive cardiomyopathy. Catheter Cardiovasc Intervent 2020;95:1240–1248. - PubMed

