A path to translation: How 3D patient tumor avatars enable next generation precision oncology
- PMID: 36270276
- PMCID: PMC10576652
- DOI: 10.1016/j.ccell.2022.09.017
A path to translation: How 3D patient tumor avatars enable next generation precision oncology
Abstract
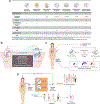
3D patient tumor avatars (3D-PTAs) hold promise for next-generation precision medicine. Here, we describe the benefits and challenges of 3D-PTA technologies and necessary future steps to realize their potential for clinical decision making. 3D-PTAs require standardization criteria and prospective trials to establish clinical benefits. Innovative trial designs that combine omics and 3D-PTA readouts may lead to more accurate clinical predictors, and an integrated platform that combines diagnostic and therapeutic development will accelerate new treatments for patients with refractory disease.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interests S.B., M.B., H.M., Z.P., C.A.M., K.M.N., M.F.P., R.C.S., K.S. and J.H.C. have no competing interests to declare. S.E.K. is an advisor to Xilis, Inc. F.M.B. declares consulting/advisory fees from AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, Daiichi Sankyo Co. Ltd., DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., Genentech Inc., Harbinger Health, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Protai Bio Ltd, Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks, Black Diamond, Biovica, Eisai, FogPharma, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Sanofi, Silverback Therapeutics, Spectrum Pharmaceuticals, and Zentalis; sponsored research to her institution from Aileron Therapeutics, Inc., AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co.; and honoraria for a speaking engagement from Chugai Biopharmaceuticals. S.K. is a consultant for FGH BioTech and has received research funding from FGH BioTech and Systems Oncology. K.A.P. is co-inventor on a patent for EGFRT790M mutation testing issued, licensed, and with royalties paid from Molecular Diagnostics/Memorial Sloan Kettering Cancer Center. She reports research funding to the institution from AstraZeneca, Roche/Genentech, Boehringer Ingelheim, and D2G Oncology and consulting for AstraZeneca and Jannssen. M.G.C. reports grants from NeoImmuneTech, as well as other support from Orbus Therapeutics, Incyte, Merck, and UpToDate outside the submitted work; in addition, M.G.C. has a patent for Zika virus strains for the treatment of glioblastoma pending. E.E.’s full disclosures are given here: www.bit.ly/3xuWMer. J.T. reports personal financial interest in form of scientific consultancy role for Array Biopharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, Sotio Biotech, Taiho, Tessa Therapeutics, and TheraMyc; and holds stocks in Oniria Therapeutics and also educational collaboration with Imedex/HMP, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER). D.A.T. is a member of the Scientific Advisory Board and receives stock options from Leap Therapeutics, Surface Oncology, Cygnal Therapeutics, Mestag Therapeutics, and Xilis, Inc. outside the submitted work. D.A.T. is scientific co-founder of Mestag Therapeutics. D.A.T. has received research grant support from Fibrogen, Mestag, and ONO Therapeutics. D.A.T. receives grant funding from the Lustgarten Foundation, the NIH, and the Thompson Foundation. None of this work is related to the publication. No other disclosures were reported. C.D.W. is a part-time consultant for LifeNet Health and receives grant funding from AACR-Novocure and Varian Medical Systems. X.S. and H.C. are cofounders of Xilis, Inc. and inventors on patents related to organoid research and micro-organospheres. X.S. is CEO of Xilis, Inc. H.C.’s full disclosure is given here: www.uu.nl/staff/JCClevers/Additionalfunctions. M.S., B.J.R., P.R., X.L., B.E.W., and A.L.W. do not report any conflicts.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

