AAV-mediated gene therapy produces fertile offspring in the Lhcgr-deficient mouse model of Leydig cell failure
- PMID: 36270285
- PMCID: PMC9729833
- DOI: 10.1016/j.xcrm.2022.100792
AAV-mediated gene therapy produces fertile offspring in the Lhcgr-deficient mouse model of Leydig cell failure
Abstract
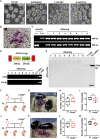
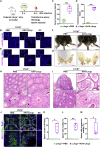
Leydig cell failure (LCF) caused by gene mutation results in testosterone deficiency and infertility. Serum testosterone levels can be recovered via testosterone replacement; however, established therapies have shown limited success in restoring fertility. Here, we use a luteinizing hormone/choriogonadotrophin receptor (Lhcgr)-deficient mouse model of LCF to investigate the feasibility of gene therapy for restoring testosterone production and fertility. We screen several adeno-associated virus (AAV) serotypes and identify AAV8 as an efficient vector to drive exogenous Lhcgr expression in progenitor Leydig cells through interstitial injection. We observe considerable testosterone recovery and Leydig cell maturation after AAV8-Lhcgr treatment in pubertal Lhcgr-/- mice. Of note, this gene therapy partially recovers sexual development, substantially restores spermatogenesis, and effectively produces fertile offspring. Furthermore, these favorable effects can be reproduced in adult Lhcgr-/- mice. Our proof-of-concept experiments in the mouse model demonstrate that AAV-mediated gene therapy may represent a promising therapeutic approach for patients with LCF.
Keywords: AAV; Leydig cell failure; fertility; gene therapy; offspring; sexual development; spermatogenesis; testosterone.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Bhasin S., Brito J.P., Cunningham G.R., Hayes F.J., Hodis H.N., Matsumoto A.M., Snyder P.J., Swerdloff R.S., Wu F.C., Yialamas M.A. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018;103:1715–1744. doi: 10.1210/jc.2018-00229. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

