Protein A-Nanoluciferase fusion protein for generalized, sensitive detection of immunoglobulin G
- PMID: 36270332
- PMCID: PMC9826736
- DOI: 10.1016/j.ab.2022.114929
Protein A-Nanoluciferase fusion protein for generalized, sensitive detection of immunoglobulin G
Abstract
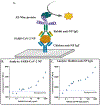
Detection and quantification of antibodies, especially immunoglobulin G (IgG), is a cornerstone of ELISAs, many diagnostics, and the development of antibody-based drugs. Current state-of-the-art immunoassay techniques for antibody detection require species-specific secondary antibodies and carefully-controlled bioconjugations. Poor conjugation efficiency degrades assay performance and increases the risk of clinical false positives due to non-specific binding. We developed a generic, highly-sensitive platform for IgG quantification by fusing the IgG-Fc binding Z domain of Staphylococcal Protein A with the ultrabright bioluminescence reporter Nanoluc-luciferase (Nluc). We demonstrated the application of this fusion protein in a sandwich IgG detection immunoassay using surface-bound antigens to capture target IgG and protein A-Nanoluc fusion as the detector. We optimized the platform's sensitivity by incorporating multiple repeats of the Z domain into the fusion protein constructs. Using rabbit and mouse anti-SARS-CoV-2 Nucleoprotein IgGs as model analytes, we performed ELISAs in two different formats, either with SARS-CoV-2 Nucleoprotein as the capture antigen or with polyclonal chicken IgY as the capture antibody. Using standard laboratory equipment, the platform enabled the quantitation of antibody analytes at concentrations as low as 10 pg/mL (67 fM).
Keywords: Bioluminescence; IgG detection; Immunoassay; Nanoluciferase; Protein A; SARS-CoV-2 Nucleoprotein.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest The authors declare no competing interests.
Figures




References
-
- Zhu L, Cui X, Wu J, Wang Z, Wang P, Hou Y, Yang M, Fluorescence immunoassay based on carbon dots as labels for the detection of human immunoglobulin G, Anal. Methods 6 (2014) 4430–4436. 10.1039/C4AY00717D. - DOI
-
- Brinkley M, A brief survey of methods for preparing protein conjugates with dyes, haptens and crosslinking reagents, Bioconjug. Chem 3 (1992) 2–13. https://pubs.acs.org/doi/10.1021/bc00013a001. - DOI - PubMed
-
- Nilsson BL, Hondal RJ, Soellner MB, Raines RT, Protein assembly by orthogonal chemical ligation methods, J. Am. Chem. Soc 125 (2003) 5268–5269. https://pubs.acs.org/doi/10.1021/ja029752e. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

