Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases
- PMID: 36270778
- PMCID: PMC10314090
- DOI: 10.1136/gutjnl-2022-327337
Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases
Abstract
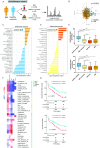
Objective: The extent to which tryptophan (Trp) metabolism alterations explain or influence the outcome of inflammatory bowel diseases (IBDs) is still unclear. However, several Trp metabolism end-products are essential to intestinal homeostasis. Here, we investigated the role of metabolites from the kynurenine pathway.
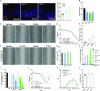
Design: Targeted quantitative metabolomics was performed in two large human IBD cohorts (1069 patients with IBD). Dextran sodium sulphate-induced colitis experiments in mice were used to evaluate effects of identified metabolites. In vitro, ex vivo and in vivo experiments were used to decipher mechanisms involved. Effects on energy metabolism were evaluated by different methods including Single Cell mEtabolism by profiling Translation inHibition.
Results: In mice and humans, intestinal inflammation severity negatively correlates with the amount of xanthurenic (XANA) and kynurenic (KYNA) acids. Supplementation with XANA or KYNA decreases colitis severity through effects on intestinal epithelial cells and T cells, involving Aryl hydrocarbon Receptor (AhR) activation and the rewiring of cellular energy metabolism. Furthermore, direct modulation of the endogenous tryptophan metabolism, using the recombinant enzyme aminoadipate aminotransferase (AADAT), responsible for the generation of XANA and KYNA, was protective in rodent colitis models.
Conclusion: Our study identified a new mechanism linking Trp metabolism to intestinal inflammation and IBD. Bringing back XANA and KYNA has protective effects involving AhR and the rewiring of the energy metabolism in intestinal epithelial cells and CD4+ T cells. This study paves the way for new therapeutic strategies aiming at pharmacologically correcting its alterations in IBD by manipulating the endogenous metabolic pathway with AADAT.
Keywords: inflammatory bowel disease.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HS report lecture fee, board membership, or consultancy from Carenity, AbbVie, Astellas, Danone, Ferring, Mayoly Spindler, MSD, Novartis, Roche, Tillots, Enterome, BiomX, Biose, Novartis,Takeda, Biocodex and is cofounder of Exeliom Biosciences.
Figures





Comment in
-
Disentangling tryptophan metabolism in inflammatory bowel diseases.Gut. 2023 Jul;72(7):1235-1236. doi: 10.1136/gutjnl-2022-328853. Epub 2023 Jan 3. Gut. 2023. PMID: 36596710 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
