Machine Learning Guides Peptide Nucleic Acid Flow Synthesis and Sequence Design
- PMID: 36270977
- PMCID: PMC9731686
- DOI: 10.1002/advs.202201988
Machine Learning Guides Peptide Nucleic Acid Flow Synthesis and Sequence Design
Abstract
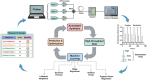
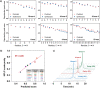
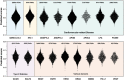
Peptide nucleic acids (PNAs) are potential antisense therapies for genetic, acquired, and viral diseases. Efficiently selecting candidate PNA sequences for synthesis and evaluation from a genome containing hundreds to thousands of options can be challenging. To facilitate this process, this work leverages machine learning (ML) algorithms and automated synthesis technology to predict PNA synthesis efficiency and guide rational PNA sequence design. The training data is collected from individual fluorenylmethyloxycarbonyl (Fmoc) deprotection reactions performed on a fully automated PNA synthesizer. The optimized ML model allows for 93% prediction accuracy and 0.97 Pearson's r. The predicted synthesis scores are validated to be correlated with the experimental high-performance liquid chromatography (HPLC) crude purities (correlation coefficient R2 = 0.95). Furthermore, a general applicability of ML is demonstrated through designing synthetically accessible antisense PNA sequences from 102 315 predicted candidates targeting exon 44 of the human dystrophin gene, SARS-CoV-2, HIV, as well as selected genes associated with cardiovascular diseases, type II diabetes, and various cancers. Collectively, ML provides an accurate prediction of PNA synthesis quality and serves as a useful computational tool for informing PNA sequence design.
Keywords: automated synthesis; drug design; machine learning; peptide nucleic acid; yield prediction.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
B.L.P. is a co‐founder and/or member of the scientific advisory board of several companies focusing on the development of protein and peptide therapeutics. All other authors declare no competing interests.
Figures


Similar articles
-
Peptide nucleic acids (PNAs) patterning by an automated microarray synthesis system through photolithography.J Nanosci Nanotechnol. 2013 Mar;13(3):2061-7. doi: 10.1166/jnn.2013.6890. J Nanosci Nanotechnol. 2013. PMID: 23755646
-
PNA-Pdx: Versatile Peptide Nucleic Acid-Based Detection of Nucleic Acids and SNPs.Anal Chem. 2023 Sep 26;95(38):14209-14218. doi: 10.1021/acs.analchem.3c01809. Epub 2023 Sep 11. Anal Chem. 2023. PMID: 37696750 Free PMC article.
-
Fmoc-Based Assembly of PNA Oligomers: Manual and Microwave-Assisted Automated Synthesis.Methods Mol Biol. 2020;2105:1-16. doi: 10.1007/978-1-0716-0243-0_1. Methods Mol Biol. 2020. PMID: 32088861
-
Peptide-nucleic acids (PNAs): a tool for the development of gene expression modifiers.Curr Pharm Des. 2001 Nov;7(17):1839-62. doi: 10.2174/1381612013397087. Curr Pharm Des. 2001. PMID: 11562312 Review.
-
Peptide nucleic acid (PNA) binding-mediated gene regulation.Cell Res. 2004 Apr;14(2):111-6. doi: 10.1038/sj.cr.7290209. Cell Res. 2004. PMID: 15115611 Review.
Cited by
-
Advance in peptide-based drug development: delivery platforms, therapeutics and vaccines.Signal Transduct Target Ther. 2025 Mar 5;10(1):74. doi: 10.1038/s41392-024-02107-5. Signal Transduct Target Ther. 2025. PMID: 40038239 Free PMC article. Review.
-
Computer vision as a new paradigm for monitoring of solution and solid phase peptide synthesis.Chem Sci. 2023 Oct 10;14(42):11872-11880. doi: 10.1039/d3sc01383a. eCollection 2023 Nov 1. Chem Sci. 2023. PMID: 37920332 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
