Structural biology of DOCK-family guanine nucleotide exchange factors
- PMID: 36271211
- PMCID: PMC10152721
- DOI: 10.1002/1873-3468.14523
Structural biology of DOCK-family guanine nucleotide exchange factors
Abstract
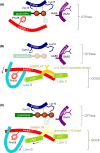
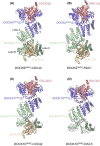
DOCK proteins are a family of multi-domain guanine nucleotide exchange factors (GEFs) that activate the RHO GTPases CDC42 and RAC1, thereby regulating several RHO GTPase-dependent cellular processes. DOCK proteins are characterized by the catalytic DHR2 domain (DOCKDHR2 ), and a phosphatidylinositol(3,4,5)P3 -binding DHR1 domain (DOCKDHR1 ) that targets DOCK proteins to plasma membranes. DOCK-family GEFs are divided into four subfamilies (A to D) differing in their specificities for CDC42 and RAC1, and the composition of accessory signalling domains. Additionally, the DOCK-A and DOCK-B subfamilies are constitutively associated with ELMO proteins that auto-inhibit DOCK GEF activity. We review structural studies that have provided mechanistic insights into DOCK-protein functions. These studies revealed how a conserved nucleotide sensor in DOCKDHR2 catalyses nucleotide exchange, the basis for how different DOCK proteins activate specifically CDC42 and RAC1, and sometimes both, and how up-stream regulators relieve the ELMO-mediated auto-inhibition. We conclude by presenting a model for full-length DOCK9 of the DOCK-D subfamily. The involvement of DOCK GEFs in a range of diseases highlights the importance of gaining structural insights into these proteins to better understand and specifically target them.
Keywords: CDC42; DOCK proteins; RAC1; guanine nucleotide exchange factors.
© 2022 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures







Similar articles
-
Multiple factors confer specific Cdc42 and Rac protein activation by dedicator of cytokinesis (DOCK) nucleotide exchange factors.J Biol Chem. 2011 Jul 15;286(28):25341-51. doi: 10.1074/jbc.M111.236455. Epub 2011 May 24. J Biol Chem. 2011. PMID: 21613211 Free PMC article.
-
Structural basis for the dual GTPase specificity of the DOCK10 guanine nucleotide exchange factor.Biochem Biophys Res Commun. 2023 Apr 23;653:12-20. doi: 10.1016/j.bbrc.2023.02.054. Epub 2023 Feb 21. Biochem Biophys Res Commun. 2023. PMID: 36848820
-
Dock6, a Dock-C subfamily guanine nucleotide exchanger, has the dual specificity for Rac1 and Cdc42 and regulates neurite outgrowth.Exp Cell Res. 2007 Feb 15;313(4):791-804. doi: 10.1016/j.yexcr.2006.11.017. Epub 2006 Dec 6. Exp Cell Res. 2007. PMID: 17196961
-
Dock-family exchange factors in cell migration and disease.Eur J Cell Biol. 2014 Oct;93(10-12):466-77. doi: 10.1016/j.ejcb.2014.06.003. Epub 2014 Jun 24. Eur J Cell Biol. 2014. PMID: 25022758 Review.
-
Structural insights into the small GTPase specificity of the DOCK guanine nucleotide exchange factors.Curr Opin Struct Biol. 2021 Dec;71:249-258. doi: 10.1016/j.sbi.2021.08.001. Epub 2021 Sep 8. Curr Opin Struct Biol. 2021. PMID: 34507037 Review.
Cited by
-
Refinement of rice blast disease resistance QTLs and gene networks through meta-QTL analysis.Sci Rep. 2024 Jul 16;14(1):16458. doi: 10.1038/s41598-024-64142-0. Sci Rep. 2024. PMID: 39013915 Free PMC article.
-
VAV2 Drives EGFR-Mediated Rac1 Responses in Prostate Cancer.Mol Cancer Res. 2025 Aug 4;23(8):684-698. doi: 10.1158/1541-7786.MCR-24-0957. Mol Cancer Res. 2025. PMID: 40183768
-
Lipid nanoparticle-encapsulated DOCK11-siRNA efficiently reduces hepatitis B virus cccDNA level in infected mice.Mol Ther Methods Clin Dev. 2024 Jun 24;32(3):101289. doi: 10.1016/j.omtm.2024.101289. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39109217 Free PMC article.
-
Mapping the MOB proteins' proximity network reveals a unique interaction between human MOB3C and the RNase P complex.J Biol Chem. 2023 Sep;299(9):105123. doi: 10.1016/j.jbc.2023.105123. Epub 2023 Aug 1. J Biol Chem. 2023. PMID: 37536630 Free PMC article.
-
ELMO2 is an essential regulator of carotid artery development.Nat Commun. 2025 Jun 2;16(1):5108. doi: 10.1038/s41467-025-60105-9. Nat Commun. 2025. PMID: 40456777 Free PMC article.
References
-
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. - PubMed
-
- Vega FM, Ridley AJ. SnapShot: rho family GTPases. Cell. 2007;129:1430. - PubMed
-
- Heasman SJ, Ridley AJ. Mammalian rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. - PubMed
-
- Vetter IR, Wittinghofer A. The guanine nucleotide‐binding switch in three dimensions. Science. 2001;294:1299–304. - PubMed
-
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

