Platelet lysate can support the development of a 3D-engineered skin for clinical application
- PMID: 36271300
- PMCID: PMC9839813
- DOI: 10.1007/s00441-022-03698-7
Platelet lysate can support the development of a 3D-engineered skin for clinical application
Abstract
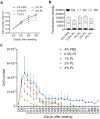
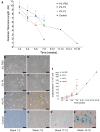
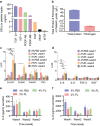
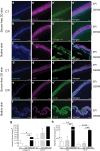
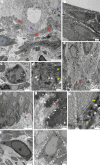
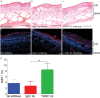
Safety concerns associated with foetal bovine serum (FBS) have restricted its translation into clinics. We hypothesised that platelet lysate (PL) can be utilised as a safe alternative to produce serum-free 3D-engineered skin. PL supported a short-term expansion of fibroblasts, with negligible replication-induced senescence and directed epidermal stratification. PL-expanded fibroblasts were phenotypically separated into three subpopulations of CD90+FAP+, CD90+FAP- and CD90-FAP+, based on CD90 (reticular marker) and FAP (papillary marker) expression profile. PL drove the expansion of the intermediate CD90+ FAP+ subpopulation in expense of reticular CD90+FAP-, which may be less fibrotic once grafted. The 3D-engineered skin cultured in PL was analysed by immunofluorescence using specific markers. Detection of ColIV and LMN-511 confirmed basement membrane. K10 confirmed near native differentiation pattern of neo-epidermis. CD29- and K5-positive interfollicular stem cells were also sustained. Transmission and scanning electron microscopies detailed the ultrastructure of the neo-dermis and neo-epidermis. To elucidate the underlying mechanism of the effect of PL on skin maturation, growth factor contents in PL were measured, and TGF-β1 was identified as one of the most abundant. TGF-β1 neutralising antibody reduced the number of Ki67-positive proliferative cells, suggesting TGF-β1 plays a role in skin maturation. Moreover, the 3D-engineered skin was exposed to lucifer yellow on days 1, 3 and 5. Penetration of lucifer yellow into the skin was used as a semi-quantitative measure of improved barrier function over time. Our findings support the concept of PL as a safe and effective serum alternative for bioengineering skin for cell therapies.
Keywords: Collagen IV; Platelet lysate; Primary keratinocytes culture; Senescence; TGF-β1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Akbarzadeh S, McKenzie MB, Rahman MM, Cleland H (2021) Allogeneic platelet-rich plasma: is it safe and effective for wound repair? Eur Surg Res 1–12 - PubMed
-
- Altran SC, Yoshito D, Isaac C, Sufi BS, Herson MR, Conceicao RO, Ferreira MC, Mathor MB. Human platelet lysate as a substitute for fetal bovine serum in human keratinocyte cell cultures conforming epithelia for transplantation. J Cell Tissue Res. 2013;13(2):3603–3609.
-
- Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991;88(2):565–569. doi: 10.1073/pnas.88.2.565. - DOI - PMC - PubMed
-
- Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schafer R, Sella S, Rodeghiero F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016;7(1):93. doi: 10.1186/s13287-016-0352-x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

