Maternal and neonatal outcomes with the use of long acting, compared to intermediate acting basal insulin (NPH) for managing diabetes during pregnancy: a systematic review and meta-analysis
- PMID: 36271431
- PMCID: PMC9585834
- DOI: 10.1186/s13098-022-00925-7
Maternal and neonatal outcomes with the use of long acting, compared to intermediate acting basal insulin (NPH) for managing diabetes during pregnancy: a systematic review and meta-analysis
Abstract
Background: To assess the impact of long-acting insulin analogues, compared to intermediate acting neutral protamine Hagedron (NPH), on maternal, perinatal and neonatal outcomes.
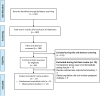
Methods: Studies for inclusion in the review were identified using a structured search strategy in PubMed, Scopus and Cochrane Central Register of Controlled Trials (CENTRAL) database. Studies that were randomized controlled trials or observational in design were considered for inclusion. Eligible studies should have compared the maternal, perinatal and neonatal outcomes between pregnant women with gestational diabetes mellitus (GDM) managed by intermediate acting (NPH) and by long-acting insulin analogues. Statistical analysis was performed using STATA software.
Results: We found 17 studies to be eligible for inclusion. The mean gestational weight gain and risk of maternal hypoglycaemia, hypertensive disorder, caesarean delivery, spontaneous abortion, endometritis and wound infection or dehiscence were similar among pregnant women with GDM managed using long-acting insulin analogues and NPH. Those receiving long-acting insulin analogues had significantly lower HbA1c values in the second (WMD - .09, 95% CI 0.12, - 0.06; N = 4) and third trimester (WMD - 0.08, 95% CI - 0.14, - 0.02; N = 12). The mean gestational age and birth weight and risk of perinatal mortality, prematurity, large for gestational age, small for gestational age, shoulder dystocia and congenital abnormalities was similar among babies in both groups. No statistically significant differences in risk of admission to neonatal intensive care unit, respiratory distress, neonatal hypoglycaemia, 5 min APGAR score of < 7, neonatal hyperbilirubinemia and sepsis was observed. The quality of pooled evidence, as per GRADE criteria, was judged to be "very low" for all the maternal and neonatal outcomes considered.
Conclusions: Findings suggest no significant differences in the maternal, perinatal and neonatal outcomes between intermediate and long-acting insulin analogues. The results provide support for use of long-acting insulin analogues in women with GDM. However, evidence is still needed from high quality randomized controlled trials to arrive at a recommendation for inclusion in routine clinical care.
Keywords: Complications; Detemir; Glargine; Intermediate acting insulin; Long-acting insulin analogues; Meta-analysis; NPH; Neonatal outcomes; Neutral protamine Hagedron; Obstetric outcomes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Detemir vs neutral protamine Hagedorn insulin for diabetes mellitus in pregnancy: a comparative effectiveness, randomized controlled trial.Am J Obstet Gynecol. 2021 Jul;225(1):87.e1-87.e10. doi: 10.1016/j.ajog.2021.04.223. Epub 2021 Apr 15. Am J Obstet Gynecol. 2021. PMID: 33865836 Clinical Trial.
-
Fetal biometry for guiding the medical management of women with gestational diabetes mellitus for improving maternal and perinatal health.Cochrane Database Syst Rev. 2019 Sep 3;9(9):CD012544. doi: 10.1002/14651858.CD012544.pub2. Cochrane Database Syst Rev. 2019. PMID: 31476798 Free PMC article.
-
Perinatal outcomes in pregnancies managed with antenatal insulin glargine.Am J Perinatol. 2009 Sep;26(8):591-5. doi: 10.1055/s-0029-1220782. Epub 2009 Apr 15. Am J Perinatol. 2009. PMID: 19370512
-
Screening and diagnosing gestational diabetes mellitus.Evid Rep Technol Assess (Full Rep). 2012 Oct;(210):1-327. Evid Rep Technol Assess (Full Rep). 2012. PMID: 24423035 Free PMC article. Review.
-
Randomized controlled trial of insulin detemir versus NPH for the treatment of pregnant women with diabetes.Am J Obstet Gynecol. 2015 Sep;213(3):426.e1-7. doi: 10.1016/j.ajog.2015.06.010. Epub 2015 Jun 9. Am J Obstet Gynecol. 2015. PMID: 26070699 Clinical Trial.
Cited by
-
Maternal Infections, Antibiotics, Steroid Use, and Diabetes Mellitus Increase Risk of Early-Onset Sepsis in Preterm Neonates: A Nationwide Population-Based Study.Pathogens. 2025 Jan 17;14(1):89. doi: 10.3390/pathogens14010089. Pathogens. 2025. PMID: 39861049 Free PMC article.
References
-
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(2):S141–146. - PubMed
-
- Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(2):S112–119. - PubMed
-
- Fetita L-S, Sobngwi E, Serradas P, Calvo F, Gautier J-F. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab. 2006;91:3718–3724. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

