Efficacy and Safety of Parathyroid Hormone Replacement With TransCon PTH in Hypoparathyroidism: 26-Week Results From the Phase 3 PaTHway Trial
- PMID: 36271471
- PMCID: PMC10099823
- DOI: 10.1002/jbmr.4726
Efficacy and Safety of Parathyroid Hormone Replacement With TransCon PTH in Hypoparathyroidism: 26-Week Results From the Phase 3 PaTHway Trial
Abstract
Conventional therapy for hypoparathyroidism consisting of active vitamin D and calcium aims to alleviate hypocalcemia but fails to restore normal parathyroid hormone (PTH) physiology. PTH replacement therapy is the ideal physiologic treatment for hypoparathyroidism. The double-blind, placebo-controlled, 26-week, phase 3 PaTHway trial assessed the efficacy and safety of PTH replacement therapy for hypoparathyroidism individuals with the investigational drug TransCon PTH (palopegteriparatide). Participants (n = 84) were randomized 3:1 to once-daily TransCon PTH (initially 18 μg/d) or placebo, both co-administered with conventional therapy. The study drug and conventional therapy were titrated according to a dosing algorithm guided by serum calcium. The composite primary efficacy endpoint was the proportion of participants at week 26 who achieved normal albumin-adjusted serum calcium levels (8.3-10.6 mg/dL), independence from conventional therapy (requiring no active vitamin D and ≤600 mg/d of calcium), and no increase in study drug over 4 weeks before week 26. Other outcomes of interest included health-related quality of life measured by the 36-Item Short Form Survey (SF-36), hypoparathyroidism-related symptoms, functioning, and well-being measured by the Hypoparathyroidism Patient Experience Scale (HPES), and urinary calcium excretion. At week 26, 79% (48/61) of participants treated with TransCon PTH versus 5% (1/21) wiplacebo met the composite primary efficacy endpoint (p < 0.0001). TransCon PTH treatment demonstrated a significant improvement in all key secondary endpoint HPES domain scores (all p < 0.01) and the SF-36 Physical Functioning subscale score (p = 0.0347) compared with placebo. Additionally, 93% (57/61) of participants treated with TransCon PTH achieved independence from conventional therapy. TransCon PTH treatment normalized mean 24-hour urine calcium. Overall, 82% (50/61) treated with TransCon PTH and 100% (21/21) wiplacebo experienced adverse events; most were mild (46%) or moderate (46%). No study drug-related withdrawals occurred. In conclusion, TransCon PTH maintained normocalcemia while permitting independence from conventional therapy and was well-tolerated in individuals with hypoparathyroidism. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Trial registration: ClinicalTrials.gov NCT04009291.
Keywords: CLINICAL TRIALS; DISORDERS OF CALCIUM/PHOSPHATE; HORMONE REPLACEMENT; PARATHYROID-RELATED DISORDERS; PTH/VIT D/FGF23.
© 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Figures



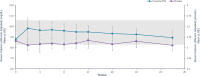


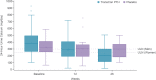
References
-
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab. 2016;101(6):2300‐2312. - PubMed
-
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab. 2016;101(6):2273‐2283. - PubMed
-
- Arlt W, Fremerey C, Callies F, et al. Well‐being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol. 2002;146(2):215‐222. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

