Mechanistic and pharmacodynamic studies of DuoBody-CD3x5T4 in preclinical tumor models
- PMID: 36271507
- PMCID: PMC9458754
- DOI: 10.26508/lsa.202201481
Mechanistic and pharmacodynamic studies of DuoBody-CD3x5T4 in preclinical tumor models
Abstract
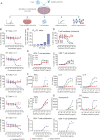
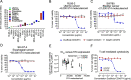
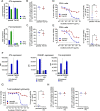
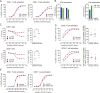
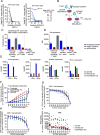
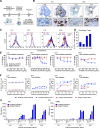
CD3 bispecific antibodies (bsAbs) show great promise as anticancer therapeutics. Here, we show in-depth mechanistic studies of a CD3 bsAb in solid cancer, using DuoBody-CD3x5T4. Cross-linking T cells with tumor cells expressing the oncofetal antigen 5T4 was required to induce cytotoxicity. Naive and memory CD4+ and CD8+ T cells were equally effective at mediating cytotoxicity, and DuoBody-CD3x5T4 induced partial differentiation of naive T-cell subsets into memory-like cells. Tumor cell kill was associated with T-cell activation, proliferation, and production of cytokines, granzyme B, and perforin. Genetic knockout of FAS or IFNGR1 in 5T4+ tumor cells abrogated tumor cell kill. In the presence of 5T4+ tumor cells, bystander kill of 5T4- but not of 5T4-IFNGR1- tumor cells was observed. In humanized xenograft models, DuoBody-CD3x5T4 antitumor activity was associated with intratumoral and peripheral blood T-cell activation. Lastly, in dissociated patient-derived tumor samples, DuoBody-CD3x5T4 activated tumor-infiltrating lymphocytes and induced tumor-cell cytotoxicity, even when most tumor-infiltrating lymphocytes expressed PD-1. These data provide an in-depth view on the mechanism of action of a CD3 bsAb in preclinical models of solid cancer.
© 2022 Kemper et al.
Conflict of interest statement
K Kemper, EN van den Brink, R Rademaker, D Verzijl, PJ Engelberts, and ECW Breij are inventors on granted or filed patents pertaining to DuoBody-CD3x5T4. All authors are employees and shareholders of Genmab. DuoBody-CD3x5T4 was co-developed by Genmab and AbbVie Inc (NCT04424641 [closed]).
Figures










References
-
- Alam SMK, Jasti S, Kshirsagar SK, Tannetta DS, Dragovic RA, Redman CW, Sargent IL, Hodes HC, Nauser TL, Fortes T, et al. (2018) Trophoblast glycoprotein (TPGB/5T4) in human placenta: Expression, regulation, and presence in extracellular microvesicles and exosomes. Reprod Sci 25: 185–197. 10.1177/1933719117707053 - DOI - PMC - PubMed
-
- Appleton KM, Elrod AK, Lassahn KA, Shuford S, Holmes LM, DesRochers TM (2021) PD-1/PD-L1 checkpoint inhibitors in combination with olaparib display antitumor activity in ovarian cancer patient-derived three-dimensional spheroid cultures. Cancer Immunol Immunother 70: 843–856. 10.1007/s00262-021-02849-z - DOI - PMC - PubMed
-
- Arenas EJ, Martinez-Sabadell A, Rius Ruiz I, Roman Alonso M, Escorihuela M, Luque A, Fajardo CA, Gros A, Klein C, Arribas J (2021) Acquired cancer cell resistance to T cell bispecific antibodies and CAR T targeting HER2 through JAK2 down-modulation. Nat Commun 12: 1237. 10.1038/s41467-021-21445-4 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
