Descending pathways from the superior colliculus mediating autonomic and respiratory effects associated with orienting behaviour
- PMID: 36271640
- PMCID: PMC10107157
- DOI: 10.1113/JP283789
Descending pathways from the superior colliculus mediating autonomic and respiratory effects associated with orienting behaviour
Abstract
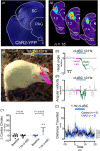
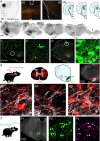
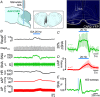
The ability to discriminate competing external stimuli and initiate contextually appropriate behaviours is a key brain function. Neurons in the deep superior colliculus (dSC) integrate multisensory inputs and activate descending projections to premotor pathways responsible for orienting, attention and defence, behaviours which involve adjustments to respiratory and cardiovascular parameters. However, the neural pathways that subserve the physiological components of orienting are poorly understood. We report that orienting responses to optogenetic dSC stimulation are accompanied by short-latency autonomic, respiratory and electroencephalographic effects in awake rats, closely mimicking those evoked by naturalistic alerting stimuli. Physiological responses were not accompanied by detectable aversion or fear, and persisted under urethane anaesthesia, indicating independence from emotional stress. Anterograde and trans-synaptic viral tracing identified a monosynaptic pathway that links the dSC to spinally projecting neurons in the medullary gigantocellular reticular nucleus (GiA), a key hub for the coordination of orienting and locomotor behaviours. In urethane-anaesthetized animals, sympathoexcitatory and cardiovascular, but not respiratory, responses to dSC stimulation were replicated by optogenetic stimulation of the dSC-GiA terminals, suggesting a likely role for this pathway in mediating the autonomic components of dSC-mediated responses. Similarly, extracellular recordings from putative GiA sympathetic premotor neurons confirmed short-latency excitatory inputs from the dSC. This pathway represents a likely substrate for autonomic components of orienting responses that are mediated by dSC neurons and suggests a mechanism through which physiological and motor components of orienting behaviours may be integrated without the involvement of higher centres that mediate affective components of defensive responses. KEY POINTS: Neurons in the deep superior colliculus (dSC) integrate multimodal sensory signals to elicit context-dependent innate behaviours that are accompanied by stereotypical cardiovascular and respiratory activities. The pathways responsible for mediating the physiological components of colliculus-mediated orienting behaviours are unknown. We show that optogenetic dSC stimulation evokes transient orienting, respiratory and autonomic effects in awake rats which persist under urethane anaesthesia. Anterograde tracing from the dSC identified projections to spinally projecting neurons in the medullary gigantocellular reticular nucleus (GiA). Stimulation of this pathway recapitulated autonomic effects evoked by stimulation of dSC neurons. Electrophysiological recordings from putative GiA sympathetic premotor neurons confirmed short latency excitatory input from dSC neurons. This disynaptic dSC-GiA-spinal sympathoexcitatory pathway may underlie autonomic adjustments to salient environmental cues independent of input from higher centres.
Keywords: arousal; cardiovascular; innate behaviours; sensorimotor integration; sympathetic.
© 2022 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures





Similar articles
-
Coordinated autonomic and respiratory responses evoked by alerting stimuli: Role of the midbrain colliculi.Respir Physiol Neurobiol. 2016 Jun;226:87-93. doi: 10.1016/j.resp.2015.10.012. Epub 2015 Nov 10. Respir Physiol Neurobiol. 2016. PMID: 26563455
-
Orienting-related eye-neck neurons of the medial ponto-bulbar reticular formation do not participate in horizontal canal-dependent vestibular reflexes of alert cats.Brain Res Bull. 1995;38(4):337-47. doi: 10.1016/0361-9230(95)00106-o. Brain Res Bull. 1995. PMID: 8535856
-
Control of Orienting Movements and Locomotion by Projection-Defined Subsets of Brainstem V2a Neurons.Curr Biol. 2020 Dec 7;30(23):4665-4681.e6. doi: 10.1016/j.cub.2020.09.014. Epub 2020 Oct 1. Curr Biol. 2020. PMID: 33007251
-
How visual inputs to the ponto-bulbar reticular formation are used in the synthesis of premotor signals during orienting.Prog Brain Res. 1989;80:159-70; discussion 127-8. doi: 10.1016/s0079-6123(08)62209-8. Prog Brain Res. 1989. PMID: 2699363 Review.
-
On the importance of the transient visual response in the superior colliculus.Curr Opin Neurobiol. 2008 Dec;18(6):544-51. doi: 10.1016/j.conb.2008.11.004. Epub 2008 Dec 6. Curr Opin Neurobiol. 2008. PMID: 19059772 Review.
Cited by
-
What Are the Functions of the Superior Colliculus and Its Involvement in Neurologic Disorders?Neurology. 2023 Apr 18;100(16):784-790. doi: 10.1212/WNL.0000000000207254. Neurology. 2023. PMID: 37068960 Free PMC article. No abstract available.
-
Acute Optogenetic Activation of the Subfornical Organ Produces Sympathetically Mediated Increases in Blood Pressure.Neuroendocrinology. 2025;115(6-7):564-575. doi: 10.1159/000545849. Epub 2025 Apr 14. Neuroendocrinology. 2025. PMID: 40222363 Free PMC article.
-
Low- and high-level coordination of orofacial motor actions.Curr Opin Neurobiol. 2023 Dec;83:102784. doi: 10.1016/j.conb.2023.102784. Epub 2023 Sep 25. Curr Opin Neurobiol. 2023. PMID: 37757586 Free PMC article. Review.
References
-
- Aicher, S. A. , Reis, D. J. , Nicolae, R. , & Milner, T. A. (1995). Monosynaptic projections from the medullary gigantocellular reticular formation to sympathetic preganglionic neurons in the thoracic spinal cord. Journal of Comparative Neurology, 363(4), 563–580. - PubMed
-
- Babic, T. , & Ciriello, J. (2004). Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventromedial medulla. Journal of Comparative Neurology, 469(3), 391–412. - PubMed
-
- Baudrie, V. , Tulen, J. H. , Blanc, J. , & Elghozi, J. L. (1997). Autonomic components of the cardiovascular responses to an acoustic startle stimulus in rats. Journal of Autonomic Pharmacology, 17(5), 303–309. - PubMed
-
- Boehnke, S. E. , & Munoz, D. P. (2008). On the importance of the transient visual response in the superior colliculus. Current Opinion in Neurobiology, 18(6), 544–551. - PubMed
-
- Bondarenko, E. , Beig, M. I. , Hodgson, D. M. , Braga, V. A. , & Nalivaiko, E. (2015). Blockade of the dorsomedial hypothalamus and the perifornical area inhibits respiratory responses to arousing and stressful stimuli. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 308(10), R816‐R822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

